Biography
I grew up in Frankenmuth, Michigan and graduated from Alma College with a B.S. in chemistry. This was followed by a short stint in Industry working as a technician for Dow Chemical. I obtained my M.S. in chemistry from Michigan State University working for Professor Odom on synthesizing, characterizing, and parameterizing the donor ability for an “X” ligand on a series of NCr(NiPr2)2X complexes. Following graduation, two years were spent working at Nike, Inc. as a material analyst running and maintaining instrumentation to perform quality control. But the allure of graduate school and the desire to solve our CO2 crisis was so great, that I sought out Indiana University of Bloomington to join Ken Caulton’s research group to explore routes to catalytically activate small molecules and recycle them into other useful starting materials.
Research Projects
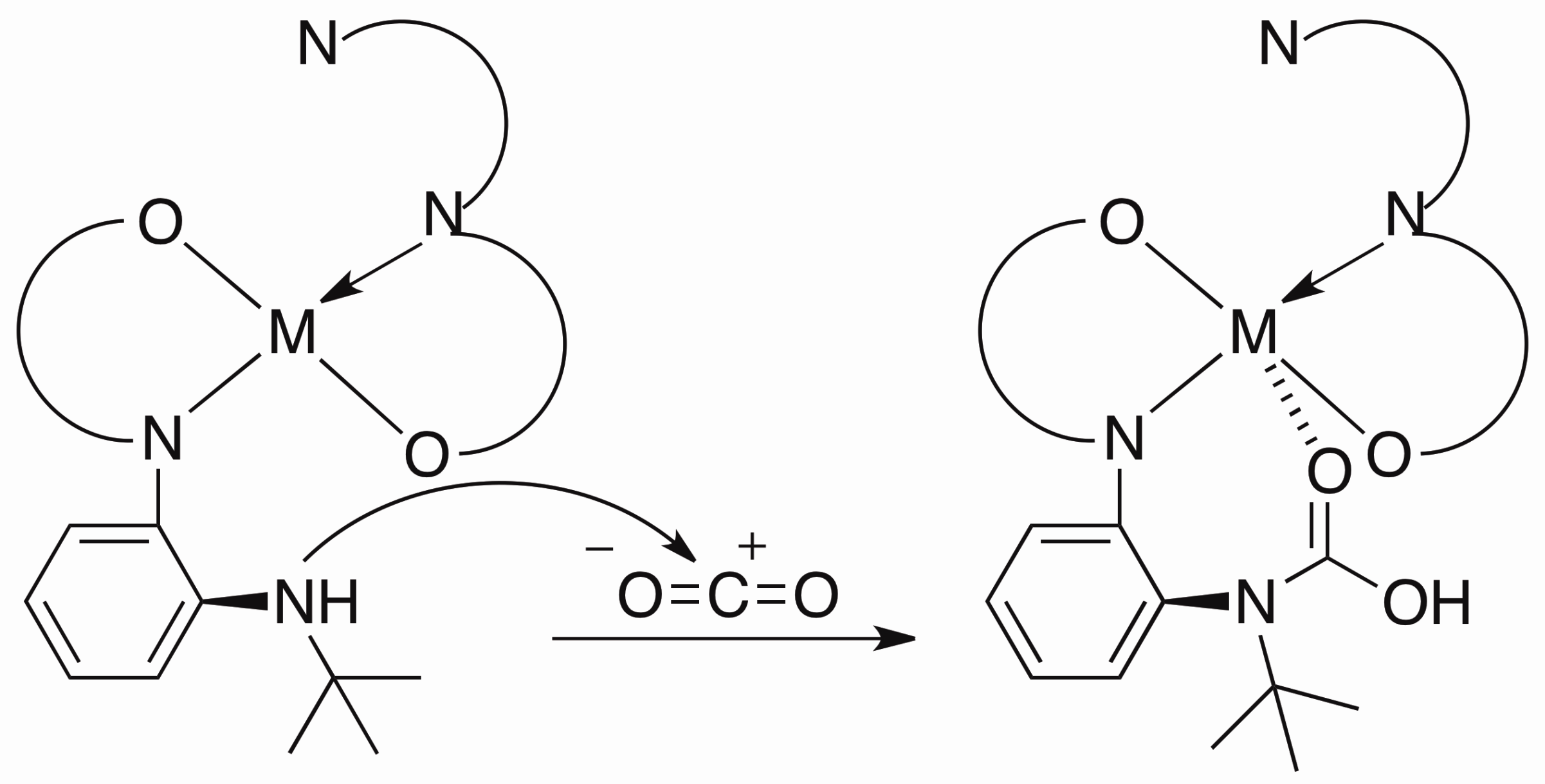
The splitting of dinitrogen into molecular nitrides provides a potential, Haber-Bosch inspired entry into N2-functionalization. Our group has a collaboration with Sven Schneider’s group in Goettingen, Germany based on a system using [{(PNP)ClMo}2-µ-N2] (PNP– = N(CH2CH2PtBu2)2–) that cleaves N2 into terminal nitrides upon protonation.1 Outlined below is my contribution to this project: to find an alternative route to synthesizing the same nitride to explore the electrophilic or nucleophilic character of the nitride nitrogen.

Refluxing W(CO)6 with HPNP in acetonitrile affords HPNPW(CO)3. N-halosuccinimide oxidizes W(0) to W(II) and deprotonates the HPNP ligand to form (PNP)W(CO)2X. We term this new synthetic method “oxidative deprotonation.”

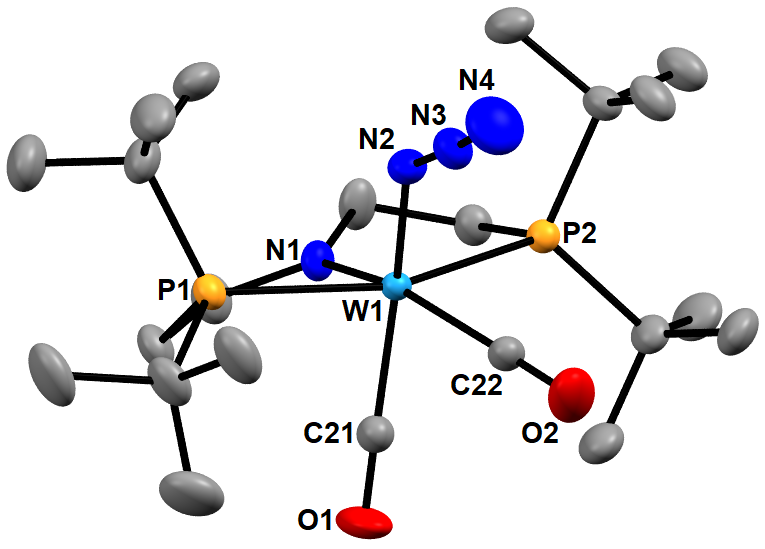
Figure 1: Mercury drawing of the nonhydrogen atoms of (PNP)W(N3)(CO)2.
Salt metathesis of (PNP)W(CO)2I with [PPN][N3] (bis(triphenylphosphine) iminium azide) forms (PNP)W(CO)2(N3) quantitatively (Figure 1). Gentle heating of (PNP)W(CO)2(N3) in benzene leads to formation of (PNP)W(N)(CO). Under 1 atm of CO gas, CO add to (PNP)W(N)(CO), reducing the metal from W(IV) to W(II) and oxidizing the carbon from +2 to +4 to form the cyanate complex (PNP)W(CO)2(NCO).

A collection of different substrates, including alkynes, dienes, isonitriles, and phosphines are being explored to interrogate reactivity preferences of our nitride.
1) Silantyev, G. A.; Förster, M.; Schluschaβ, Abbenseth, J.; Würtele, C.; Volkmann, C. Holthausen, M. C.; Schneider, S. Angew. Chem. Int. Ed. 2017, 56, 5872-5876

Biography
My hometown is Richmond, Utah in beautiful Cache Valley. I received a B.S. degree in chemistry from Utah State University (2014). While at Utah State, I studied under Dr. John Hubbard (organometallics) and Dr. Yujie Sun (water oxidation catalysts). My interests and hobbies, outside of chemistry, include: backcountry skiing in Utah powder, traditional climbing, golfing and reading. I chose Indiana University because of the outstanding friendliness of the department, the moderate size of research groups, the opportunity to collaborate, and CO2 activation research in the Caulton Group.
Research Projects
A central pillar to my research goals and philosophy is the elucidation of fundamental principles and its meaningful application to 21st century national and global problems. We face a twofold problem: satisfying rising energy demands without increasing anthropogenic climate change and conserving carbon-based resources. To address conservation of carbon-based resources, we choose the challenge of CO2 reduction to value-added, multi-carbon products.
Frontier research in this field proposes, as an attractive alternative to precious metals, redox-active ligands to assist first-row transition metals in participating in 2e– reactions by acting as an electron storage site. My current work is centered on [CrL]2 which features two divalent chromium atoms supported by a potentially redox-active pincer ligand, bis-(pyrazolate)pyridine, L2−. My goal is to exploit the reducing power (both native and when chemically reduced with KC8) and the ligand electrophile-responsiveness (proton and alkali metal) in substrate transformations. Indeed, [CrL]2 when treated with KC8 in a 1:2.3 mole ratio promptly reacts with excess CO2 to yield K10L8Cr8(CO3)4Cl2 + 4 CO which is an aggregate of the [K2Cr2L2(μ-CO3)] building block.
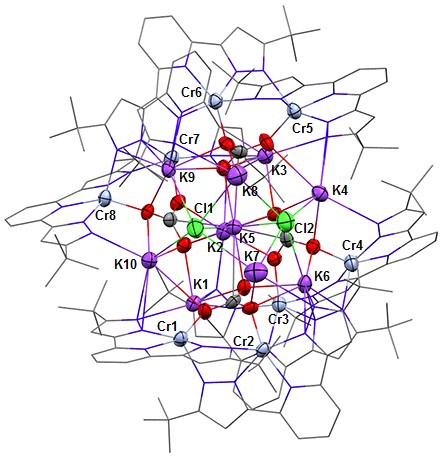
Figure 1. ORTEP drawing of K10L8Cr8(CO3)4Cl2 viewed nearly down the idealized C2 axis (K2-K5 line). All red atoms are CO2 derived oxygen.
Recently, I have extended the built-in reducing power and Brønsted basicity of [CrL]2 to bond scission or bond reduction of N=N double bonds in the substrate. I seek to develop the general principles that govern when [CrL]2 directs two-electrons to one substrate or one-electron to two substrate ligands coupled with the influence of alkali metal on the overall transformation. The reduced substrate will then bind to the newly generated Cr(III) in order to satisfy coordination number demands; this may allow for the capture of “intermediate” products, i.e., before protonation occurs, in either a chromium monomer or dimer. Ultimately, this study can potentially contribute understanding to N-O bond scission in hydroxylamine along the nitrogen cycle as nitrogen oxyanions are converted back to ammonia or dinitrogen.

Biography
I am a native of Springfield, MO where I graduated from Missouri State University with my B.S in Chemistry, 2011, and M.S. in Chemistry, 2013. My undergraduate advisor was Dr. Mark Richter under whom I studied electrogenerated chemiluminescence of Ru-chelate complexes, and I obtained my Master’s degree under the tutelage of Dr. Nikolay Gerasimchuk, with a thesis titled “Synthesis and Characterization of the First Non-Chelating Bis-Cyanoximes and their Metal Complexes.” The approach was to use easily ionizable bifunctional ligands to link together Cu(II) and Ni(II) chelates to form MOF-like structures. A desire to expand my skills as a synthetic inorganic chemist led me to Indiana University to join the Caulton group, working on catalysis with first-row transition metal complexes bearing redox non-innocent pincer ligands.
Research Projects
In the short term, my immediate research goals are the synthesis and characterization of monomeric low-valent first-row transition metal complexes of the redox non-innocent pincer 2,6-bis(6-methyl-1,2,4,5-tetrazine)yl pyridine, abbreviated btzp, specifically those of V and Fe, with, as a long term goal, a subsequent evaluation of catalytic abilities of those complexes. Characterization is done by a variety of experimental methods (direct-injection mass spectrometry, X-ray crystallography, NMR spectroscopy, and others) for structure determination and computational methods for electronic structure and to examine the effect of ligands on btzp’s pi-acidic behavior.
An approach I take to my research is to use DFT and experiment in conjunction with one another to not only explain but to some extent to predict chemical behavior. One reaction of particular interest is that of btzp with anhydrous FeCl2 (Scheme 1), with the DFT-predicted product in color.
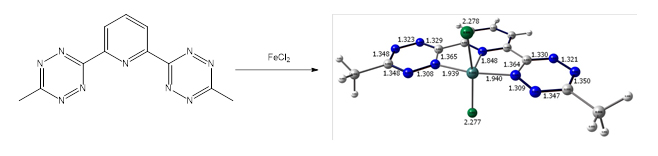
This is predicted to be a stable 16-electron compound (likely with a solvent molecule filling the remaining coordination site trans to the pyridyl), NMR spectroscopy tells a different story. The elucidation of this product is a goal of my research in the Caulton lab.
DFT has been used to probe chemical behavior, specifically the competition between btzp and other pi-acidic ligands such as CO for electron density in a reduce metal complex. This was probed by vibrational calculations on my target complex (btzp)Fe(CO)2, shown below.
This was compared to the analogous terpyridyl complex and CO stretching frequencies were shown to be lower for the terpy complex, symptomatic of weaker pi acidity of terpy compared to btzp.
Current and future work is the synthesis and isolation of monomeric (btzp)FeA2, where A is a monoanion, with a goal of isolating three-coordinate (btzp)Fe and a subsequent evaluation of its catalytic behavior.

Biography
I was born and raised in Anhui province, southern China, and received my Bachelor of Science degree in Applied Chemistry from Beijing University of Chemical Technology in 2011. When I was an undergraduate student, I worked with Dr. Haijun Hao on the study of synthesis and reactivity of Zinc Hydride compound [HC(CMeNAr)2}Zn(μ-H)]2 (Ar = 2,6-Me2C6H3)] and complexes with Zn-Zn single bond.
Research Projects
Use of carbon-based fuels has caused an increasing concentration of carbon dioxide in the atmosphere that has been continuously rising. Carbon dioxide is the most abundant green house gas, but its utilization is confronting several technical barriers. Therefore, exploring catalysts that could effectively capture CO2and convert it into valuable products has become an exciting topic in the past decades. Interestingly, nature uses Rubsico – the world’s most abundant enzyme – to capture CO2from the air and converts it into the carbon ultimately contained in our food, the fuels we use, and clothes we wear.
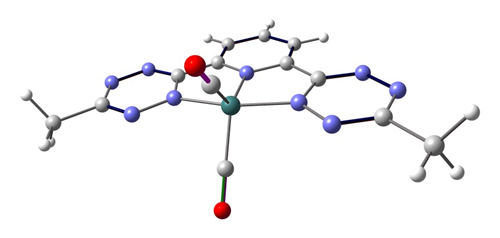
My project is focused on the study of the synthesis and reactivity of redox active ligands. We have already synthesized the iron and cobalt complexes with this newo-imino-semiquinonate ligand.
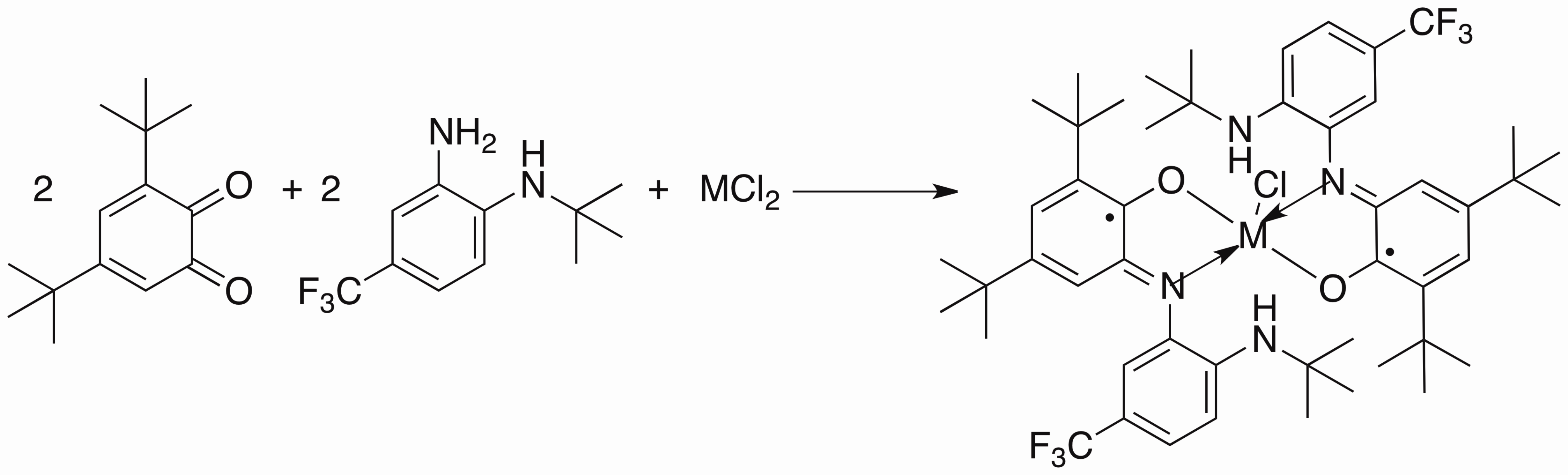
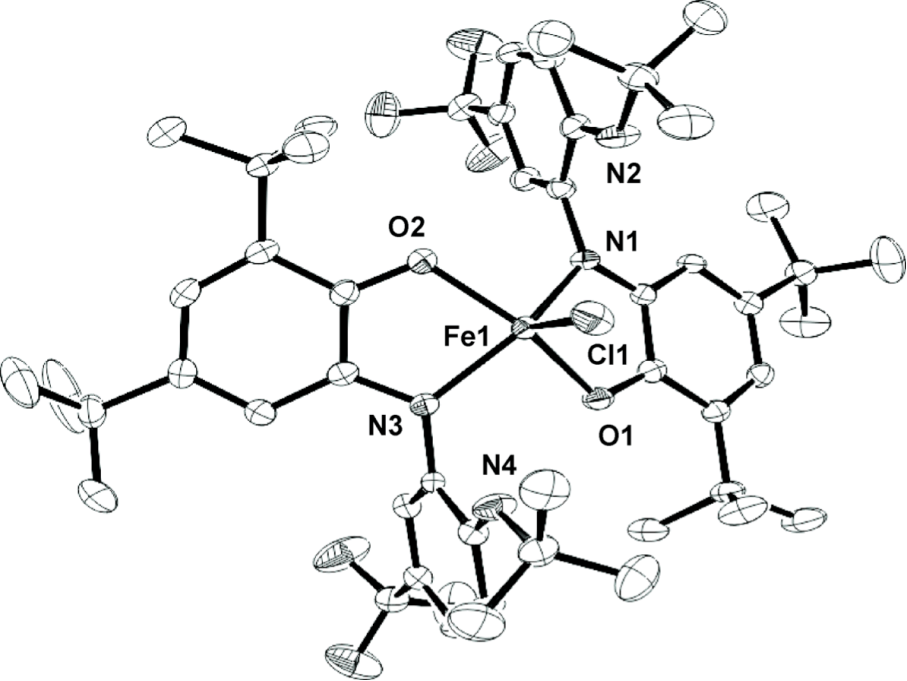
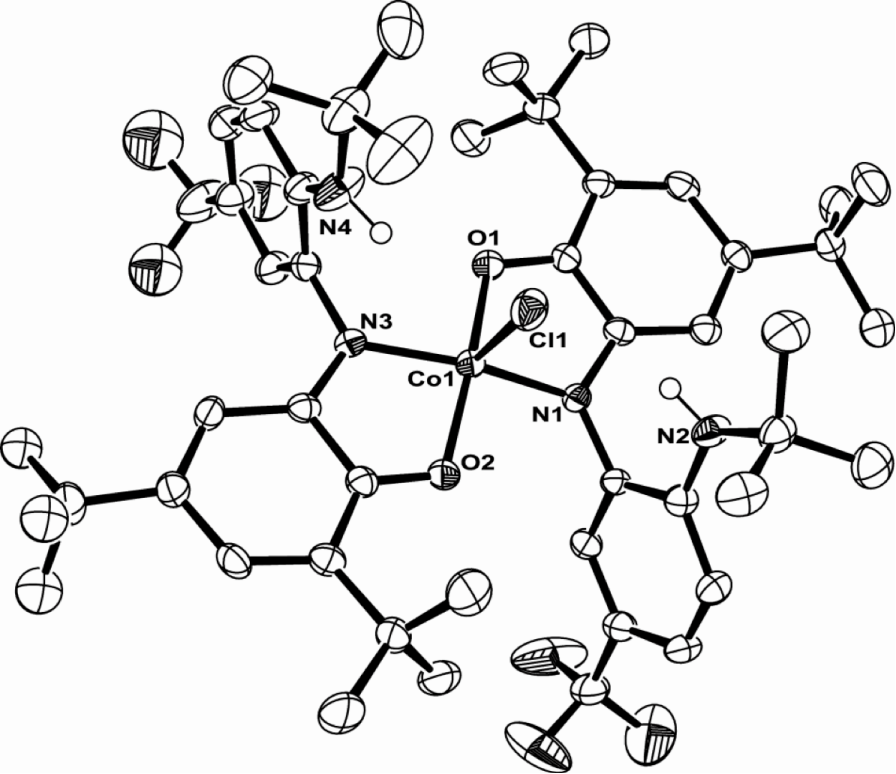
Treated with appropriate reducing agent, the chlorine in the complex above could be removed from the center metal to form a new planar compound and the ligand in this product is redox non-innocent.
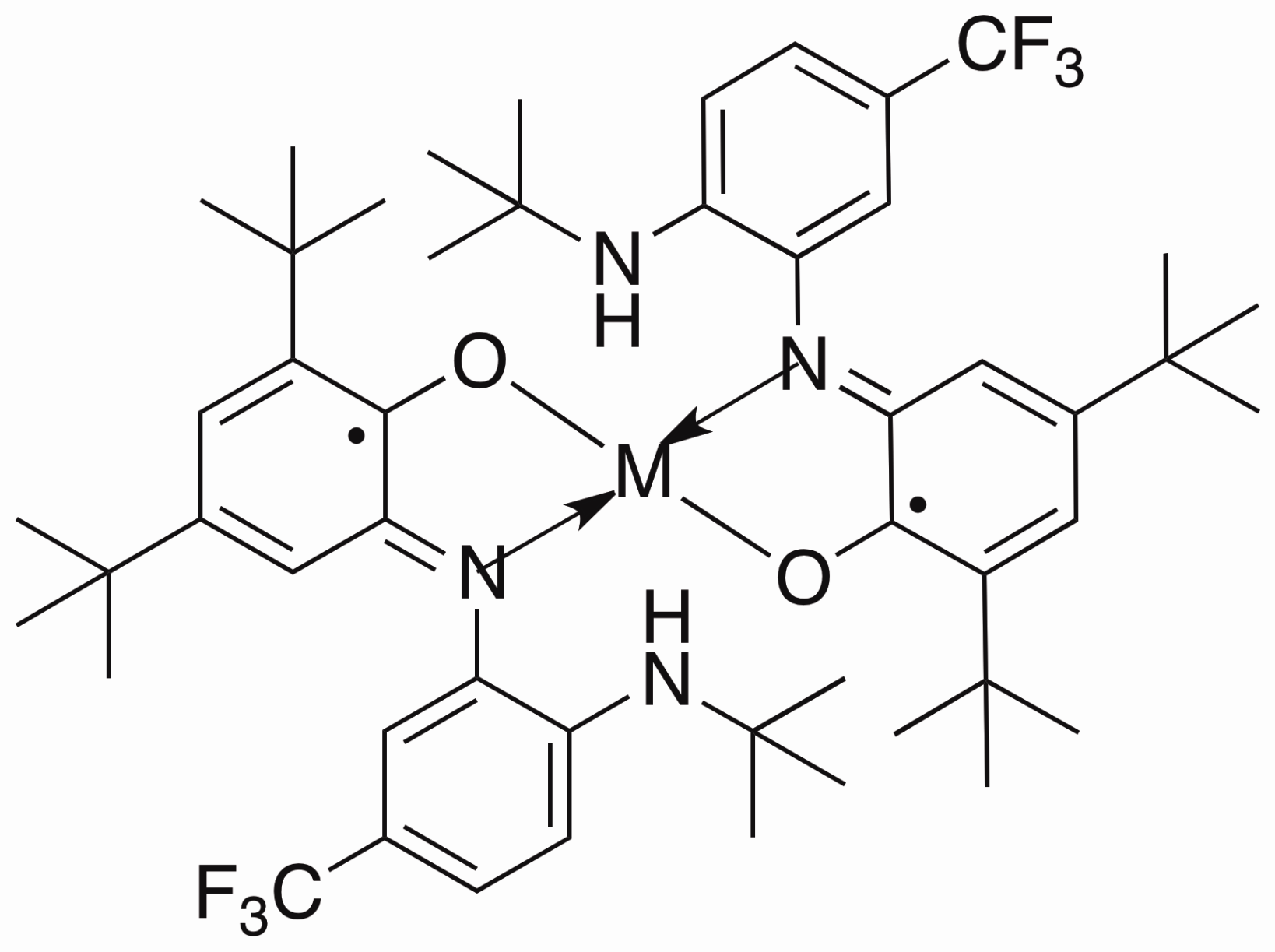
I believe that such a ligand and metal system can be used as Rubsico mimics. The CO2can be captured by the amine hanging out the system and further stabilized by coordination to the metal center. Therefore I can convert the CO2in the air into more interesting chemicals.