Biography
I grew up in Frankenmuth, Michigan and graduated from Alma College with a B.S. in chemistry. This was followed by a short stint in Industry working as a technician for Dow Chemical. I obtained my M.S. in chemistry from Michigan State University working for Professor Odom on synthesizing, characterizing, and parameterizing the donor ability for an “X” ligand on a series of NCr(NiPr2)2X complexes. Following graduation, two years were spent working at Nike, Inc. as a material analyst running and maintaining instrumentation to perform quality control. But the allure of graduate school and the desire to solve our CO2 crisis was so great, that I sought out Indiana University of Bloomington to join Ken Caulton’s research group to explore routes to catalytically activate small molecules and recycle them into other useful starting materials.
Research Projects
The splitting of dinitrogen into molecular nitrides provides a potential, Haber-Bosch inspired entry into N2-functionalization. Our group has a collaboration with Sven Schneider’s group in Goettingen, Germany based on a system using [{(PNP)ClMo}2-µ-N2] (PNP– = N(CH2CH2PtBu2)2–) that cleaves N2 into terminal nitrides upon protonation.1 Outlined below is my contribution to this project: to find an alternative route to synthesizing the same nitride to explore the electrophilic or nucleophilic character of the nitride nitrogen.

Refluxing W(CO)6 with HPNP in acetonitrile affords HPNPW(CO)3. N-halosuccinimide oxidizes W(0) to W(II) and deprotonates the HPNP ligand to form (PNP)W(CO)2X. We term this new synthetic method “oxidative deprotonation.”

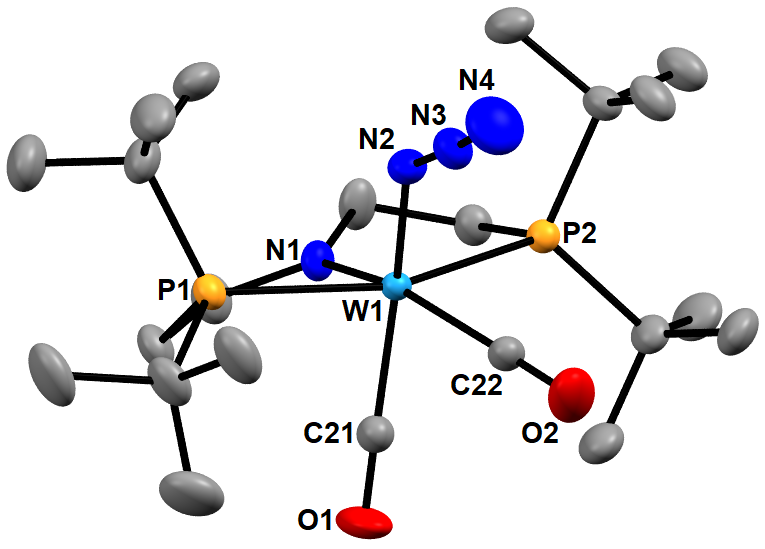
Figure 1: Mercury drawing of the nonhydrogen atoms of (PNP)W(N3)(CO)2.
Salt metathesis of (PNP)W(CO)2I with [PPN][N3] (bis(triphenylphosphine) iminium azide) forms (PNP)W(CO)2(N3) quantitatively (Figure 1). Gentle heating of (PNP)W(CO)2(N3) in benzene leads to formation of (PNP)W(N)(CO). Under 1 atm of CO gas, CO add to (PNP)W(N)(CO), reducing the metal from W(IV) to W(II) and oxidizing the carbon from +2 to +4 to form the cyanate complex (PNP)W(CO)2(NCO).

A collection of different substrates, including alkynes, dienes, isonitriles, and phosphines are being explored to interrogate reactivity preferences of our nitride.
1) Silantyev, G. A.; Förster, M.; Schluschaβ, Abbenseth, J.; Würtele, C.; Volkmann, C. Holthausen, M. C.; Schneider, S. Angew. Chem. Int. Ed. 2017, 56, 5872-5876

