Overview
Nature manipulates electrons to accomplish many needed conversions. A broad range of chemical reactions involve change of formal oxidation state; indeed, these are the reactions which have the highest energy yield, and have the potential to power human endeavor. There was a time when chemists sought the perfect transition metal for some redox transformation, based on metal oxidation state and reducing or oxidizing power. The next generation of researchers turned to “ligand design,” through the use of steric and electronic “guidance,” to impose special reactivity characteristics on those preferences intrinsic to the transition metal. This represented a new dimension, or a new variable to “engineer” desired reactivity. Indeed, this was the underlying motivation of our years of working with the “pincer” ligand N(SiMe2CH2PtBu2)2-1, since the amide “pushed” much electron density onto an attached metal, thereby making such a metal either more strongly reducing, or improving the thermodynamics of reaching an unusually high formal metal oxidation state; steric bulk in this PNP pincer anion also enables us to reach exceptionally highly unsaturated, low coordination number, metal complexes.
In most recent times, it is an appealing idea that redox equivalents, either oxidizing or reducing, can be engineered into a ligand to do still more “tuning” of the overall reactivity of a metal/ligand combination. This follows an earlier generation of Redox Active Ligands: quinones or dithiolenes, initially puzzled chemists due to the fact that the charge on a redox active
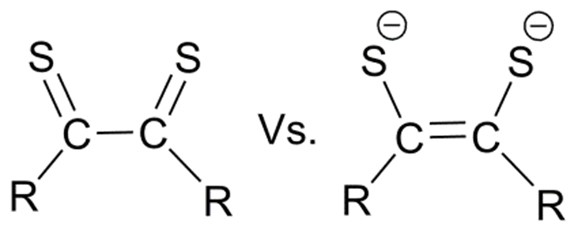
ligand could not be reliably assessed as if they were invariant, as is true of chloride or acetate, etc.: two or even three charge states are possible for catecholate or semiquinone or quinone ligands, leading to them being called “redox non-innocent.”
Current Projects
I have asked my research group members to describe their work independently in links available here and below, but I begin with an overview description of some of our research themes, which are intended to show extraordinary properties and chemical reactivity which can be designed into metal complexes from all around the periodic table. One focus is driven by both practical and fundamental thrusts: we try to elicit exceptional reactivity from certain 3d transition elements (e.g. V, Cr, Fe, Co, Ni, Cu…) since their abundance and modest cost (vs. 4d and 5d metals) means that our successes may be more rapidly adopted in practice and thus have greatest impact.
On-Surface Synthesis of RAL Complexes
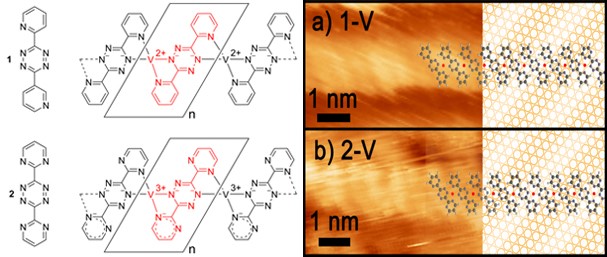
Tetrazines with appended nitrogen heterocycles can be reacted with (highly reactive, highly reducing!) metal atoms in metal “islands” previously laid down on inert surfaces to form complexes whose oxidation states can be assessed using XPS both of the metal, but also of the nitrogens: if the tetrazine is reduced, the XPS energy to remove an electron from that electron rich nitrogen core orbital decreases. Using the tetrazines illustrated below, assisted with STM to image individual molecules on the surface, we have established different geometries for platinum complexes of different N-heterocycle oxidants, and we are investigating a few very different metals (e.g. vanadium, below) as a preliminary to study of these surface synthesized molecules with CH4, CO2 and N2O. This is a collaboration with IU Prof Steven Tait and his students Chris Tempas, Toby Morris, Miao Wang and David Wisman, and delivers new understanding to the meeting place between homogeneous and heterogeneous catalysis. For more, read the link of Jake Huerfano.
Reactive Nitrides by Metal Azide Photolysis
Terminal “nitride” ligands can, in certain cases of extreme oxidation state, be sufficiently reactive that they can insert that N into H-C(sp3) bonds, converting an alkane into an amide/amine. Such reactivity, providing a way to convert alkanes into functionalized products, is thermodynamically favorable by ~ 50 kcal/mol from our DFT calculations on reaction of RN3 with C2H6. The question now is “what is the mechanism of proto-production of LMN from LMN3, and what characteristics of M will enhance the alkane functionalization by such MN?” Long lived MN3 species already in hand are photosensitive to form MN + N2, and Nick Maciulis is working to optimize that conversion, then the reactivity of the resulting nitride, complemented by DFT studies of Daniel Beagan. This work also impacts our efforts to reduce nitrate all the way to nitride, then functionalize that nitride.
Nitrogen Oxyanion Reductive Catalysis
We also address a totally new but highly environmentally relevant challenge: reduction of nitrate to lower oxidation state nitrogen compounds, catalyzed by proton responsive ligands such as our bis-pyrazolyl pyridines. The origin of the problem is that excessive ammonia fertilization of farm fields everywhere suffers fully 40% loss of nitrogen to soil organisms, with production of water soluble nitrate, which runs off farm fields into rivers and lakes, causing “dead zones” where algae grows out of control and depletes dissolved oxygen. If existing technology could trap the runoff nitrate before it accumulates in poorly mixed bodies of water like Lake Erie, the Gulf of Mexico, Monterrey Bay, and the coastal zones around Korea and Japan, then it becomes attractive to recycle that nitrogen, a terrestrial resource, back to nucleophilic nitrogen which can be incorporated into organic synthesis, including into nitrogen heterocycles that might serve as improved agricultural fertilizers. Since the Haber-Bosch process, which makes ammonia fertilizer, has huge energy requirements and also produces stoichiometric quantities of the undesirable byproduct carbon dioxide, recycling of nitrogen and reduced use of ammonia would have desirable impact on food production, water quality, and energy demands of feeding the planet.
This translates into devising earth abundant metal catalysts which can reduce NO3-1 and also break N/O bonds, the latter considerably facilitated by delivery of protons from a nearby storage site on the ligand in the metal catalyst. Proton responsive ligands thus again are appealing. Reducing equivalents required for this reductive catalysis are envisioned to come from photovoltaic devices, whose energy source is our sun. This targets energetic sustainability.
To this end, different group members are working with a broad variety of redox-active ligands, some of which also carry acidic hydrogen. Daniel Beagan is working with chromium and our tetrazine pincer ligand, anticipating that both metal and pincer can store electrons. Alyssa Cabelof is working with iron and cobalt complexes of a unique unsymmetrically armed pincer ligand with the intent of taking the metals to oxidation state +1 and below. Alec Erny is learning from Alyssa Cabelof how to synthesize her new multifunctional pincer ligand and synthesize its manganese complex to deoxygenate nitrate. Jake Huerfano has accepted the challenge of building bridges between heterogeneous and homogeneous catalysis, synthesizing a redox-active but fourfold symmetric imino-quinone which can form 2D layers on inert supports where an arriving elemental transition metal multiply reduces the “ligand;” this assembly is then studied to reduce substrate with our planar four coordinate surface species, in our collaboration with the Tait group. Our on-surface spectroscopic methods include scanning tunneling microscopy, to image arrays with molecular resolution, and XPS, to learn oxidation states of metal, ligand and substrate atoms. In parallel, Jake will form complexes of the same metals by solution synthetic methods, then reduce these for homogeneous catalytic applications.
In the hands of Jake, Dan and Alyssa, we have learned much more about “reductive silylation” and its analog, reductive borylation, both for removal of oxygen from nitrogen oxyanions. A new project by Bao Tran seeks to functionalize nitrogen at the nitride oxidation level: transfer of one or two boryl groups to coordinated nitride. Since nitride is a candidate product from N2 reduction, Bao’s work has multiple potential impacts.
Jake Huerfano’s general interest in linking together two metals for the multi-metal activation of substrate has lead him, together with colleague Jeremy Smith, to design a new ligand which fuses two N heterocyclic carbene ligands into a single entity which directs the two carbene lone pairs towards two metals at a M/M distance suitable for multi-point activation of nitrate or even azide substrate. New student Cole Seager has taken up the challenge of synthesizing this ligand and then installing two earth-abundant metals for reactivity studies. He details his work here.
Graduate student Sarah Braley seeks to develop an electrocatalytic reduction of nitrate using trivalent chromium complexes. Catalytic candidates have a macrocyclic ligand chosen to have the potential for hydrogen bonding to substrate nitrate near the redox active chromium, and the impact of the stereochemistry of the ligand around kinetically inert Cr(III) is under examination. In her work, she has discovered a remarkable role played by the electrode material.
We thus seek to take new ideas and realize these as the catalysts of tomorrow, based on rational catalyst design.
Applied Computational Chemistry – Another “Tool” in the Lab
Most of the above experimental discoveries stimulate questions about mechanism and about favorable vs. unfavorable reaction enthalpies or about whether a particular observed isomeric structure is a kinetic or thermodynamic product. In other words, we benefit from access to energetics of overall reaction enthalpies, as well as activation energies for competing alternative mechanistic paths. These we get from Density Functional Theory (DFT) calculations of most stable molecular structure and reaction energies. Following DFT calculations, we analyze wave functions, orbital compositions and energy patterns, spin densities (of radicals) and calculated vibrational frequencies, all for their chemical bonding implications. Since certain of these reactions involve changed spin state, these become challenging computations to model reliably, and here we benefit from consultations with IU Prof. Krishnan Raghavachari and students. Communicating, in plain language, between experimental and computational chemists is one skill that we emphasize, as well as a healthy skepticism of both experimental and computational “results.” Alyssa Cabelof came to IU with experience in DFT calculations, and continues here to use it as one arrow in her arsenal of research tools.
The COVID 19 pandemic put unwelcome, unprecedented, and unanticipated demands on everyone for survival, and the Caulton group was no exception. In the case of Ken, working from home became a kind of test run for retirement, which perhaps made it slightly less painful than for all other members of the research group. We kept intellectually alert and stimulated by communication with nitrogen catalysis experts across the planet: Zoom talks, either by the faculty principal investigator or their students. This relaxed and informal communication medium was educational in a bidirectional and international manner.
At the end of that experience, it became possible for Ken to reconnect with family members, who had adapted in their own creative way, resulting in a new grandchild, Lilia. Shown below is a rare success at Ken calming her, at age 7 weeks. May she grow up in a world slightly less threatened by eutrophication, climate change, rising sea levels, and health threats from her fellow citizens, all mitigated because of constructive efforts by scientists, engineers, health experts, and wise government elected by citizens who finally could convert from polarization into a negotiated compromise.

Epilog
Over the years of pursuing various potentially societally relevant research themes, what is the collective wisdom we have derived? The following illustration delivers folksy philosophy against the background of T-shirts died with Hawaiian “Original Red Dirt.” This is Hawaiian volcanic soil obtained on a research visit to an agricultural experimental farm of one corporate American fertilizer giant on the island of Kauai. In truth, some of the ideas listed can diminish the frustration of experiment research, and they warrant serious consideration. Maybe Pacific Islanders have some Truths to enlighten we arrogant scientists!

But then at the end, at the ultimate bottom line, in our study of the nitrate cycle and its human abuse, what has Caulton become ? –

Good wishes and most sincere thanks to all who threw their efforts into joining our research enterprise over the decades!
