Biography
I am a native of Springfield, MO where I graduated from Missouri State University with my B.S in Chemistry, 2011, and M.S. in Chemistry, 2013. My undergraduate advisor was Dr. Mark Richter under whom I studied electrogenerated chemiluminescence of Ru-chelate complexes, and I obtained my Master’s degree under the tutelage of Dr. Nikolay Gerasimchuk, with a thesis titled “Synthesis and Characterization of the First Non-Chelating Bis-Cyanoximes and their Metal Complexes.” The approach was to use easily ionizable bifunctional ligands to link together Cu(II) and Ni(II) chelates to form MOF-like structures. A desire to expand my skills as a synthetic inorganic chemist led me to Indiana University to join the Caulton group, working on catalysis with first-row transition metal complexes bearing redox non-innocent pincer ligands.
Research Projects
In the short term, my immediate research goals are the synthesis and characterization of monomeric low-valent first-row transition metal complexes of the redox non-innocent pincer 2,6-bis(6-methyl-1,2,4,5-tetrazine)yl pyridine, abbreviated btzp, specifically those of V and Fe, with, as a long term goal, a subsequent evaluation of catalytic abilities of those complexes. Characterization is done by a variety of experimental methods (direct-injection mass spectrometry, X-ray crystallography, NMR spectroscopy, and others) for structure determination and computational methods for electronic structure and to examine the effect of ligands on btzp’s pi-acidic behavior.
An approach I take to my research is to use DFT and experiment in conjunction with one another to not only explain but to some extent to predict chemical behavior. One reaction of particular interest is that of btzp with anhydrous FeCl2 (Scheme 1), with the DFT-predicted product in color.
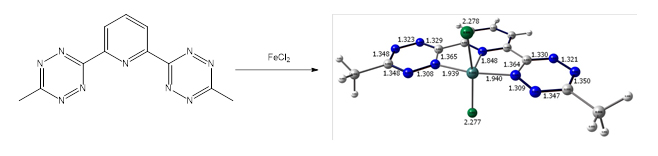
This is predicted to be a stable 16-electron compound (likely with a solvent molecule filling the remaining coordination site trans to the pyridyl), NMR spectroscopy tells a different story. The elucidation of this product is a goal of my research in the Caulton lab.
DFT has been used to probe chemical behavior, specifically the competition between btzp and other pi-acidic ligands such as CO for electron density in a reduce metal complex. This was probed by vibrational calculations on my target complex (btzp)Fe(CO)2, shown below.
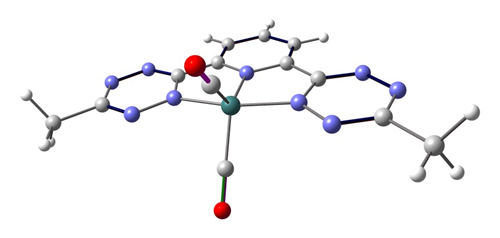
This was compared to the analogous terpyridyl complex and CO stretching frequencies were shown to be lower for the terpy complex, symptomatic of weaker pi acidity of terpy compared to btzp.
Current and future work is the synthesis and isolation of monomeric (btzp)FeA2, where A is a monoanion, with a goal of isolating three-coordinate (btzp)Fe and a subsequent evaluation of its catalytic behavior.

