Biography
I was born and raised in Anhui province, southern China, and received my Bachelor of Science degree in Applied Chemistry from Beijing University of Chemical Technology in 2011. When I was an undergraduate student, I worked with Dr. Haijun Hao on the study of synthesis and reactivity of Zinc Hydride compound [HC(CMeNAr)2}Zn(μ-H)]2 (Ar = 2,6-Me2C6H3)] and complexes with Zn-Zn single bond.
Research Projects
Use of carbon-based fuels has caused an increasing concentration of carbon dioxide in the atmosphere that has been continuously rising. Carbon dioxide is the most abundant green house gas, but its utilization is confronting several technical barriers. Therefore, exploring catalysts that could effectively capture CO2and convert it into valuable products has become an exciting topic in the past decades. Interestingly, nature uses Rubsico – the world’s most abundant enzyme – to capture CO2from the air and converts it into the carbon ultimately contained in our food, the fuels we use, and clothes we wear.
My project is focused on the study of the synthesis and reactivity of redox active ligands. We have already synthesized the iron and cobalt complexes with this newo-imino-semiquinonate ligand.
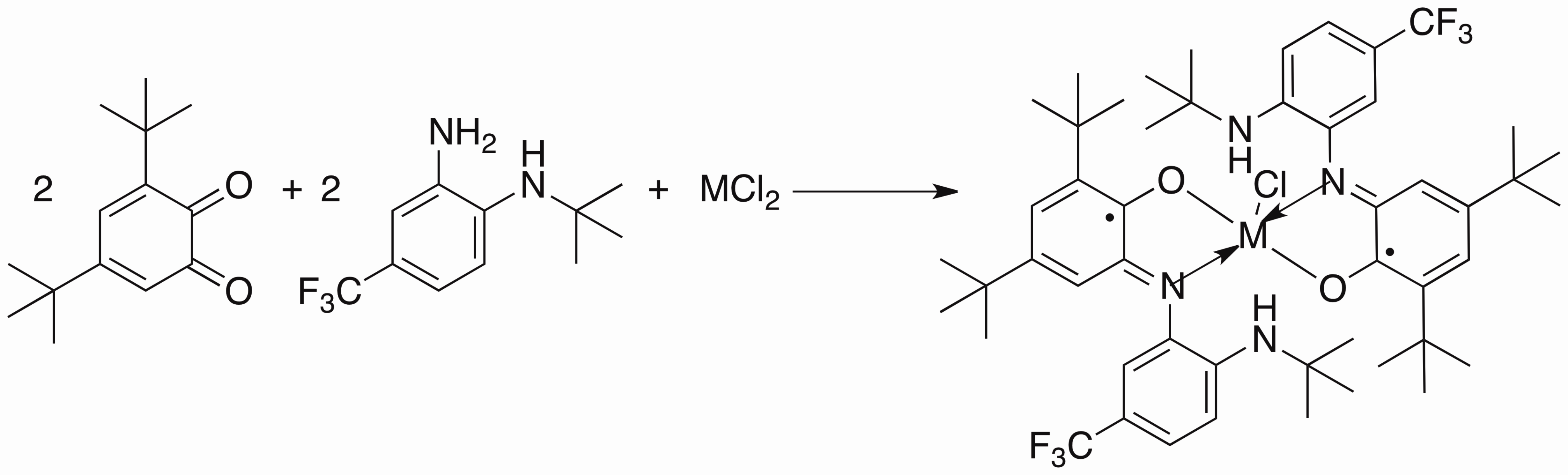
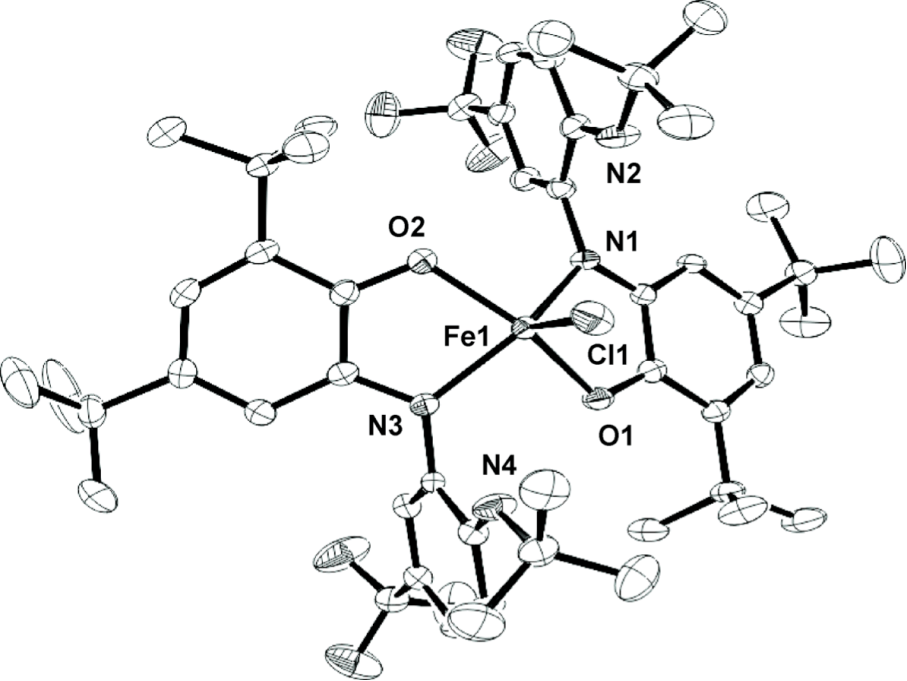
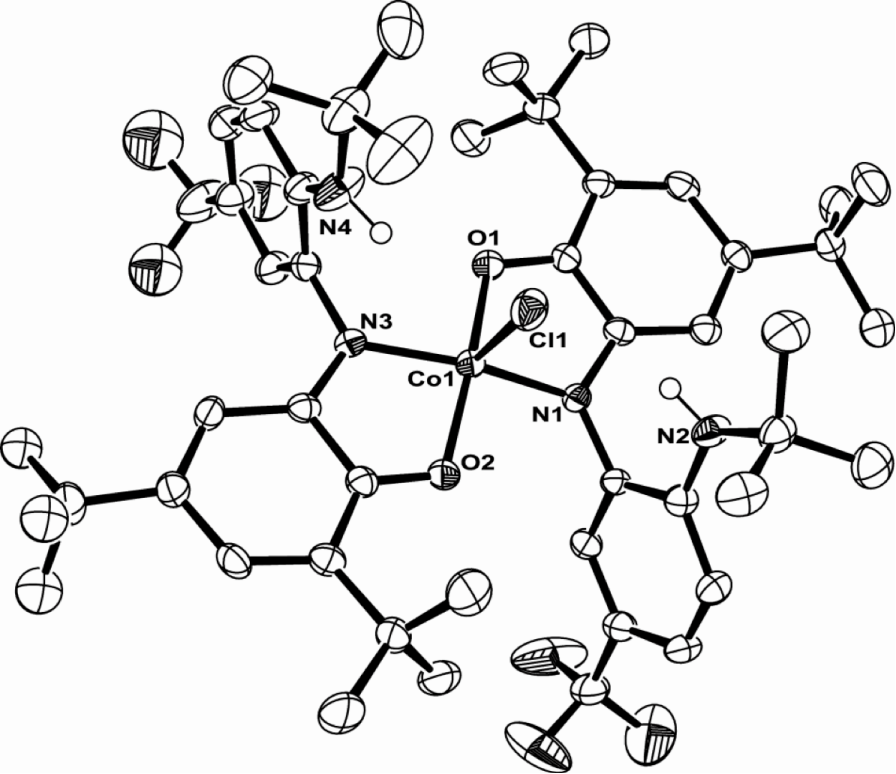
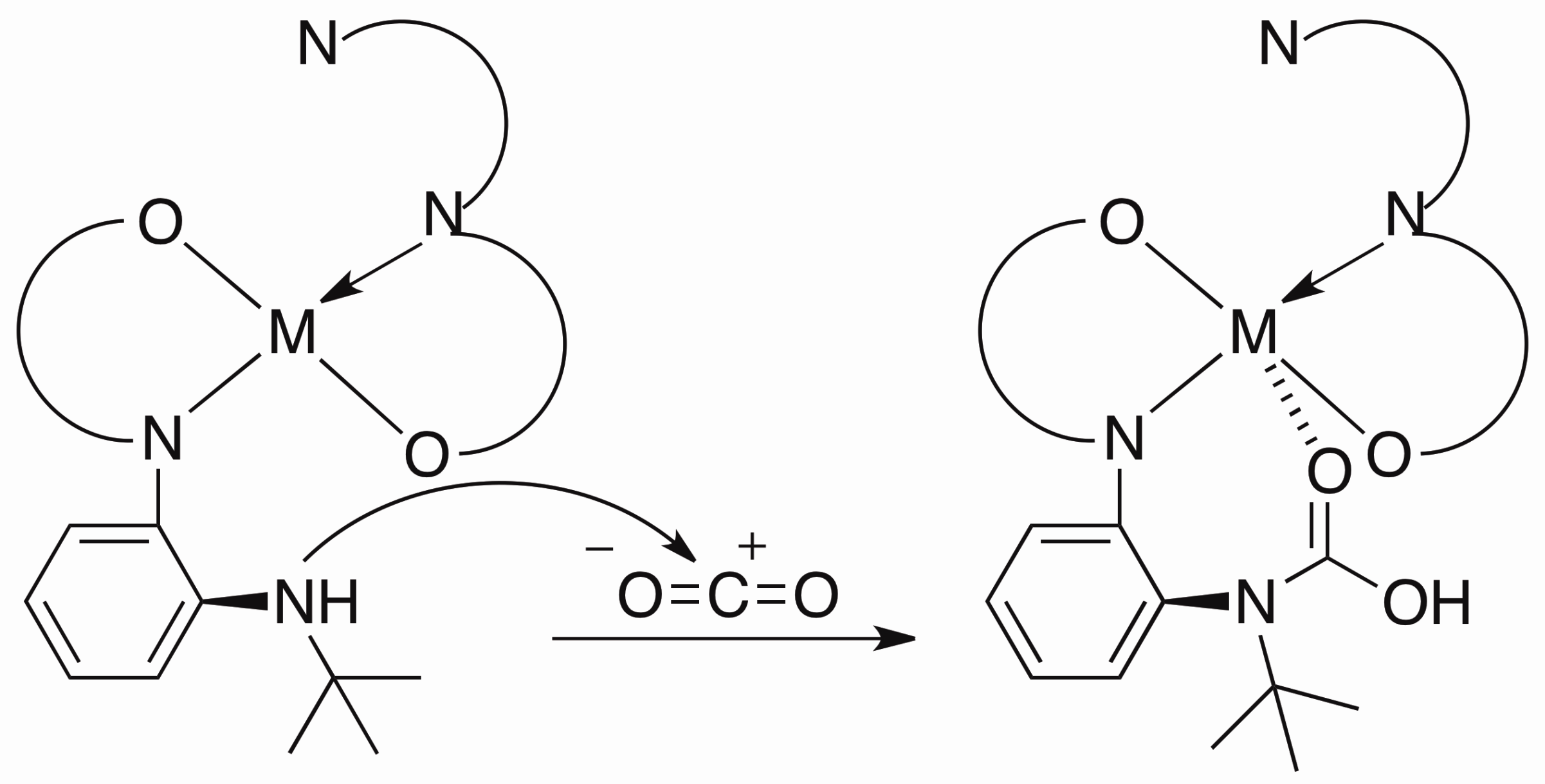
Treated with appropriate reducing agent, the chlorine in the complex above could be removed from the center metal to form a new planar compound and the ligand in this product is redox non-innocent.
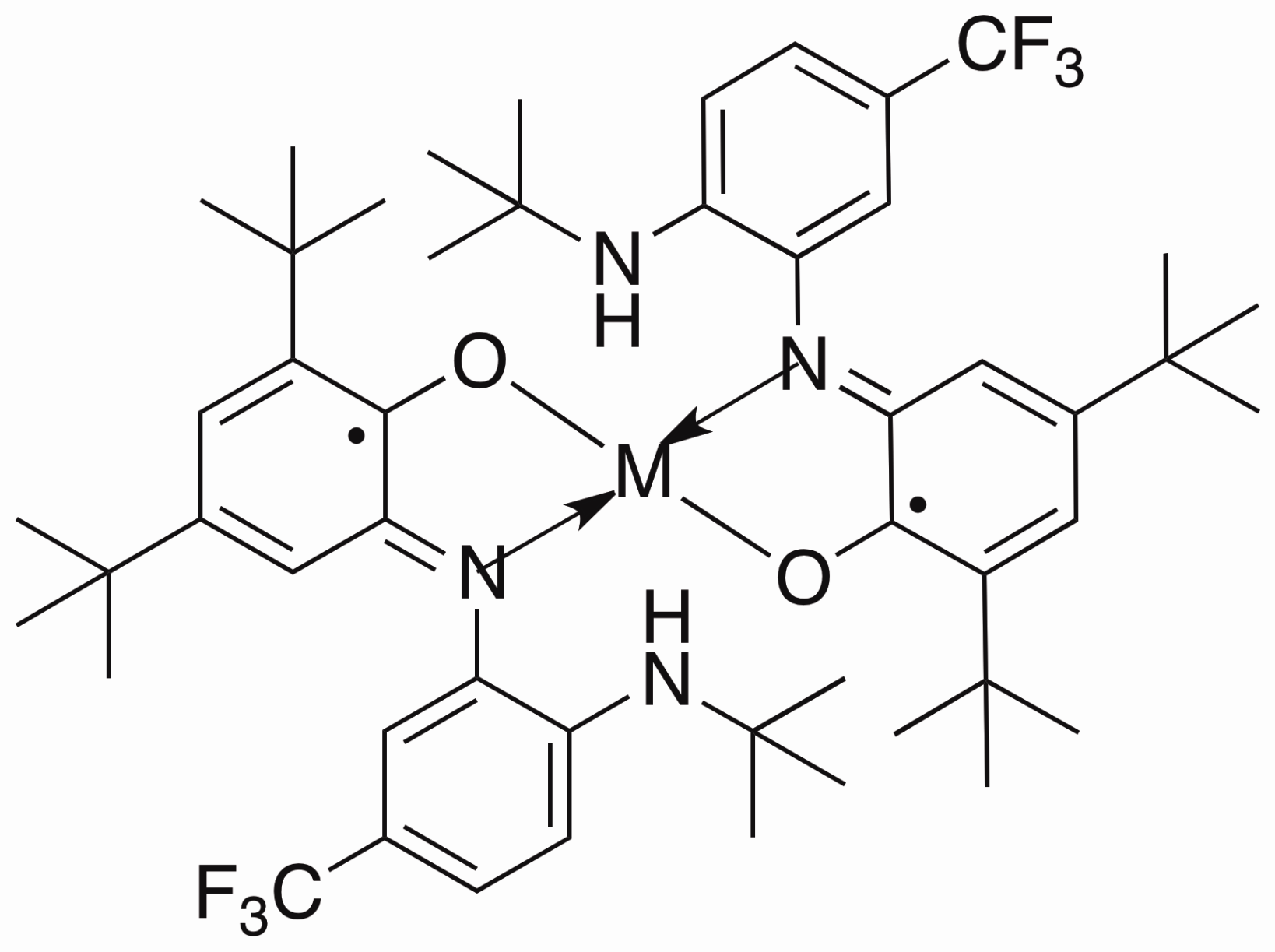
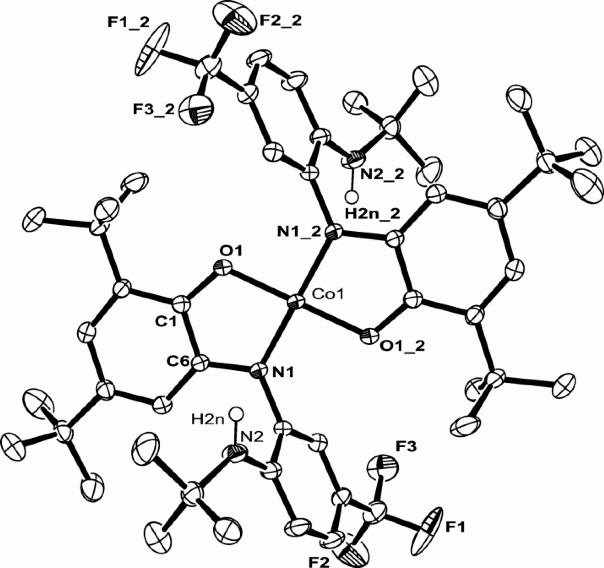
I believe that such a ligand and metal system can be used as Rubsico mimics. The CO2can be captured by the amine hanging out the system and further stabilized by coordination to the metal center. Therefore I can convert the CO2in the air into more interesting chemicals.