Biography
My hometown is Richmond, Utah in beautiful Cache Valley. I received a B.S. degree in chemistry from Utah State University (2014). While at Utah State, I studied under Dr. John Hubbard (organometallics) and Dr. Yujie Sun (water oxidation catalysts). My interests and hobbies, outside of chemistry, include: backcountry skiing in Utah powder, traditional climbing, golfing and reading. I chose Indiana University because of the outstanding friendliness of the department, the moderate size of research groups, the opportunity to collaborate, and CO2 activation research in the Caulton Group.
Research Projects
A central pillar to my research goals and philosophy is the elucidation of fundamental principles and its meaningful application to 21st century national and global problems. We face a twofold problem: satisfying rising energy demands without increasing anthropogenic climate change and conserving carbon-based resources. To address conservation of carbon-based resources, we choose the challenge of CO2 reduction to value-added, multi-carbon products.
Frontier research in this field proposes, as an attractive alternative to precious metals, redox-active ligands to assist first-row transition metals in participating in 2e– reactions by acting as an electron storage site. My current work is centered on [CrL]2 which features two divalent chromium atoms supported by a potentially redox-active pincer ligand, bis-(pyrazolate)pyridine, L2−. My goal is to exploit the reducing power (both native and when chemically reduced with KC8) and the ligand electrophile-responsiveness (proton and alkali metal) in substrate transformations. Indeed, [CrL]2 when treated with KC8 in a 1:2.3 mole ratio promptly reacts with excess CO2 to yield K10L8Cr8(CO3)4Cl2 + 4 CO which is an aggregate of the [K2Cr2L2(μ-CO3)] building block.
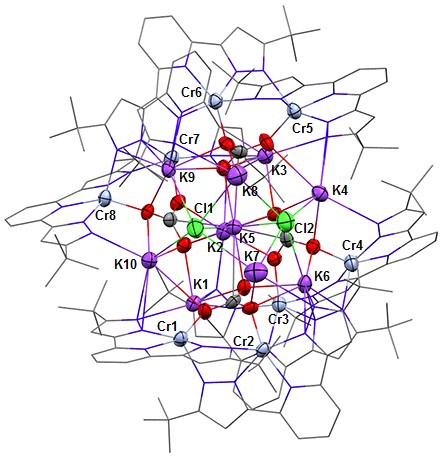
Figure 1. ORTEP drawing of K10L8Cr8(CO3)4Cl2 viewed nearly down the idealized C2 axis (K2-K5 line). All red atoms are CO2 derived oxygen.
Recently, I have extended the built-in reducing power and Brønsted basicity of [CrL]2 to bond scission or bond reduction of N=N double bonds in the substrate. I seek to develop the general principles that govern when [CrL]2 directs two-electrons to one substrate or one-electron to two substrate ligands coupled with the influence of alkali metal on the overall transformation. The reduced substrate will then bind to the newly generated Cr(III) in order to satisfy coordination number demands; this may allow for the capture of “intermediate” products, i.e., before protonation occurs, in either a chromium monomer or dimer. Ultimately, this study can potentially contribute understanding to N-O bond scission in hydroxylamine along the nitrogen cycle as nitrogen oxyanions are converted back to ammonia or dinitrogen.

