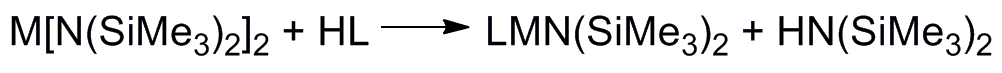Alice Hui
Biography
I am originally from Concord, North Carolina, and received my bachelor of science degree in chemistry from the University of North Carolina at Chapel Hill in 2009. As an undergrad, I worked for Dr. Royce Murray, synthesizing various ligands and metal complexes in preparation for electrochemical studies.
Research Projects
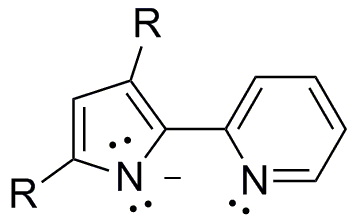
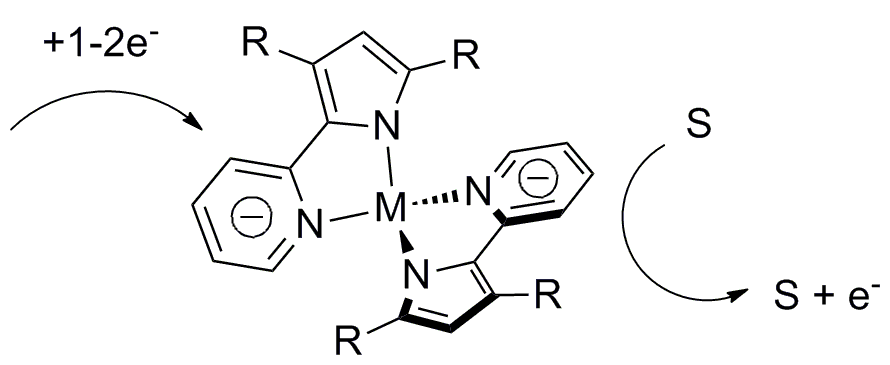
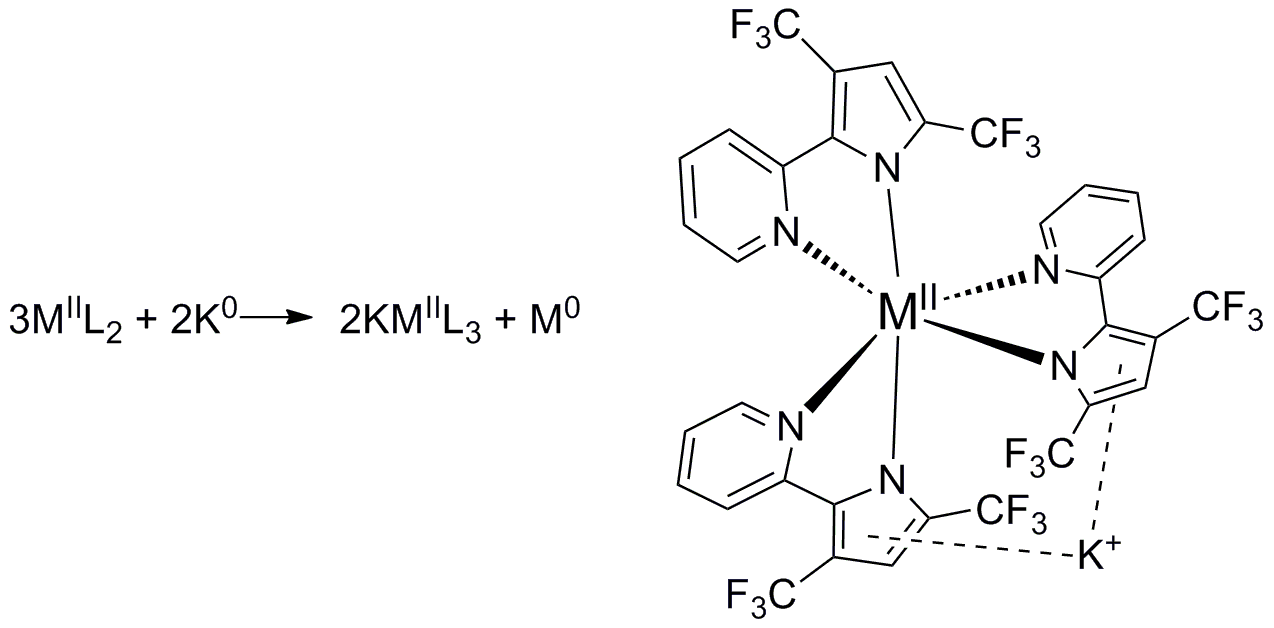
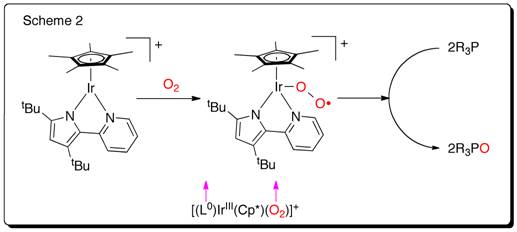
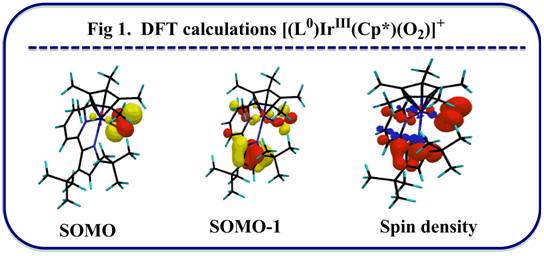
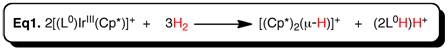
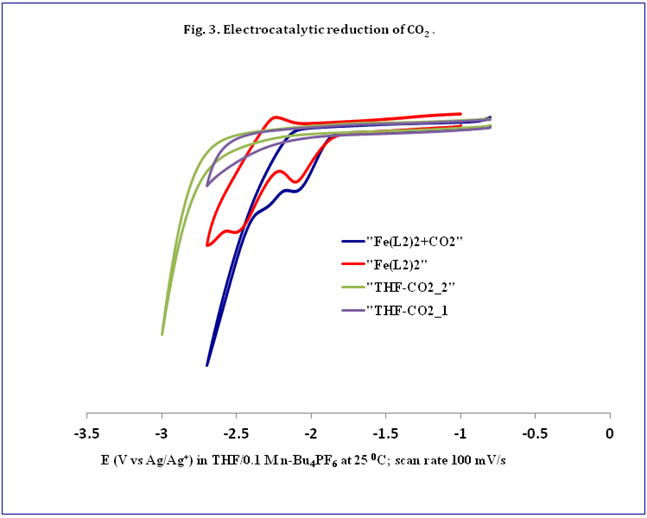
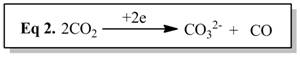
The ultimate goal of my project is to install a new type of non-innocent ligand on unsaturated metals thus creating a new type of unsaturated metal complexes to explore reactivity with small substrates like N2, CO, CO2,and H2.
1,2,4,5-s-tetrazines have been demonstrated as redox active ligands that can store up to 2 electrons. A new type of redox active ligand is the bis(tetrazinyl)pyridine ligand (bTzP) which incorporatestwo tetrazines.
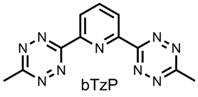
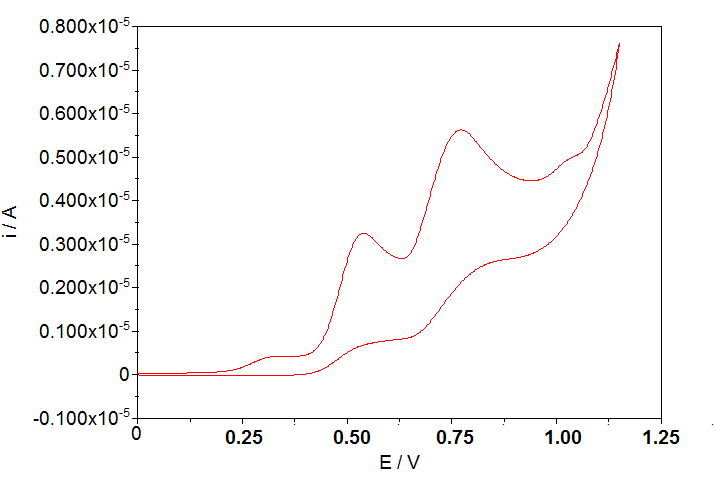
Preliminary results of reaction of bTzP and Cu powder have shown the oxidation of Cu(0) powder to Cu(II) via Electron Paramagnetic Resonance Spectroscopy (EPR) indicating that the bTzP ligand can indeed oxidize metallic copper. The question that still remains is whether there are two ligands per Cu or only one?
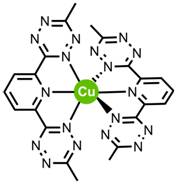
Currently, the redox properties of this pincer ligand are being explored with Ni metal. Group 10 metals are known for their abilities to activate substrates in catalytic reactions. A motivation to study nickel catalysis chemistry is simply that nickel, in comparison to other metals in the same group (Ni, Pd, Pt), is cheaper and more abundant.
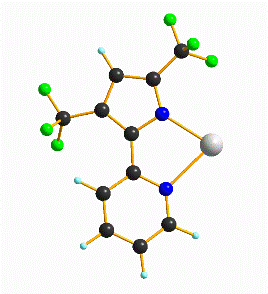
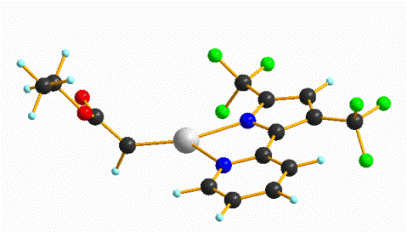
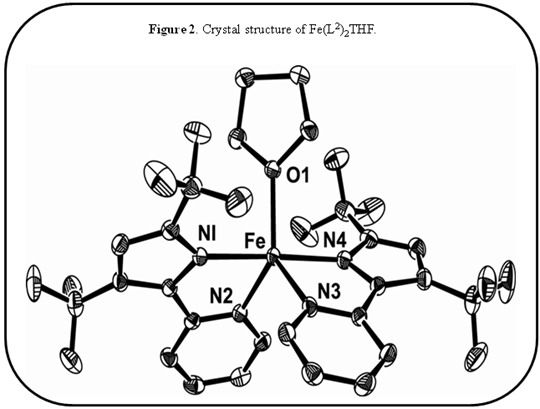
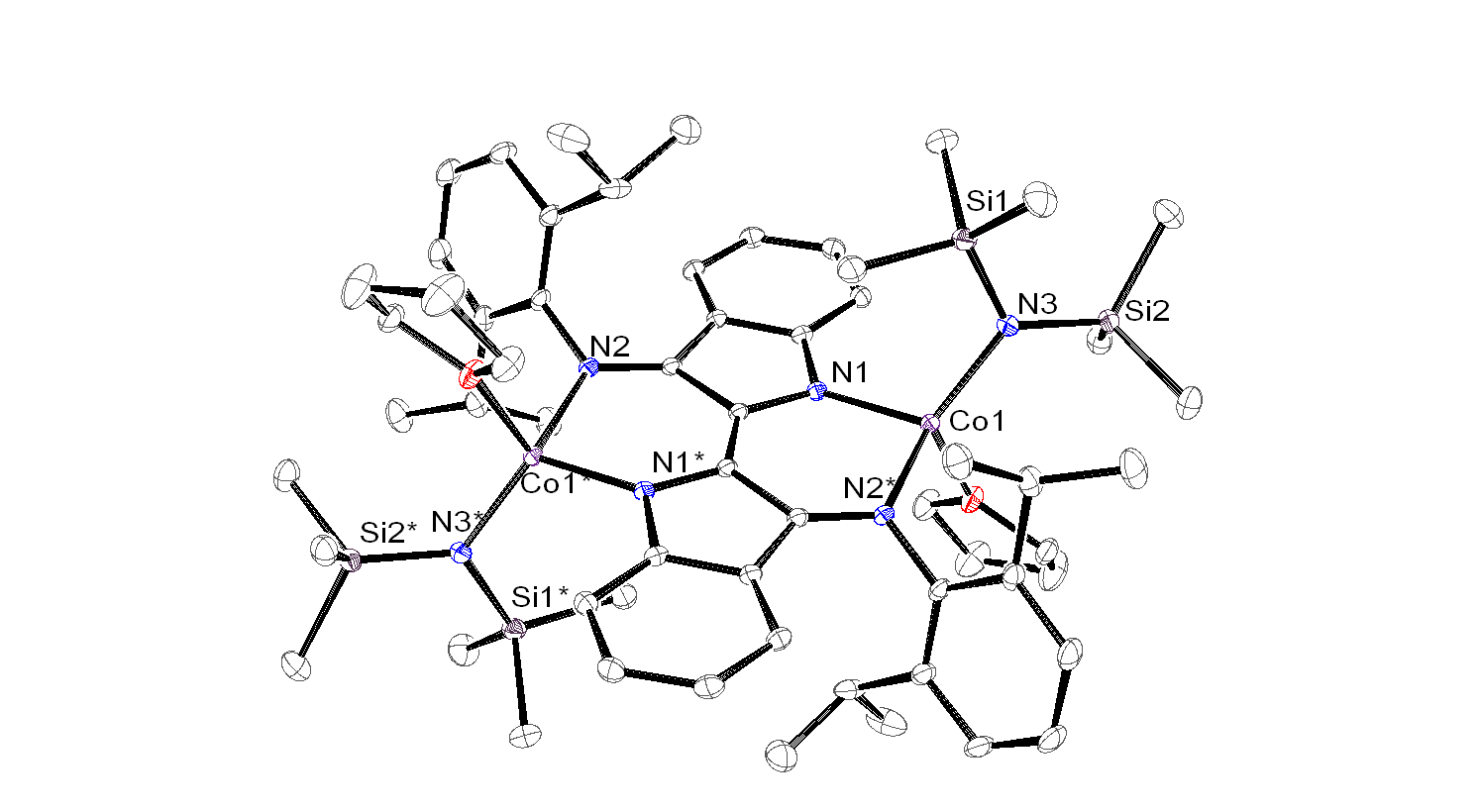
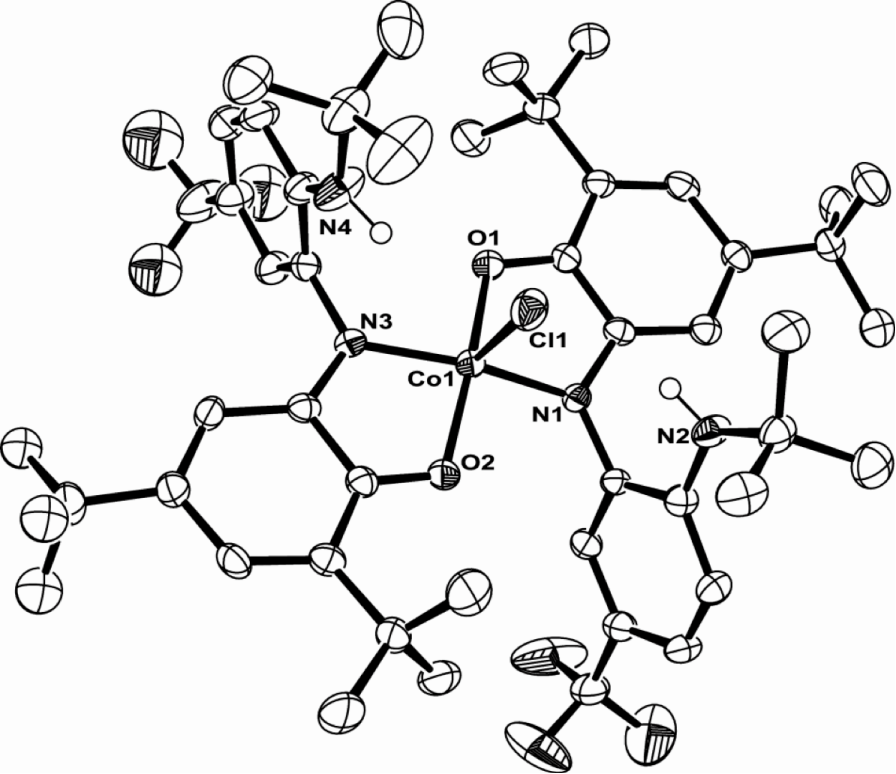
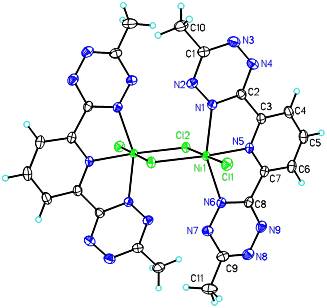
A synthetic route was explored via NiCl2·THF1.5and bTzP. The product crystallized in dichloromethane by slow diffusion of pentane to yield bright orange crystals with an empirical formula of Ni2(bTzP)2Cl4, which exists as a centrosymmetric chloride-bridged dimer as seen below.
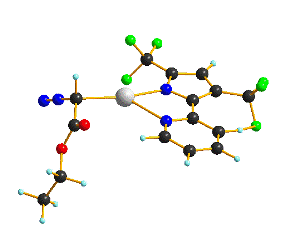
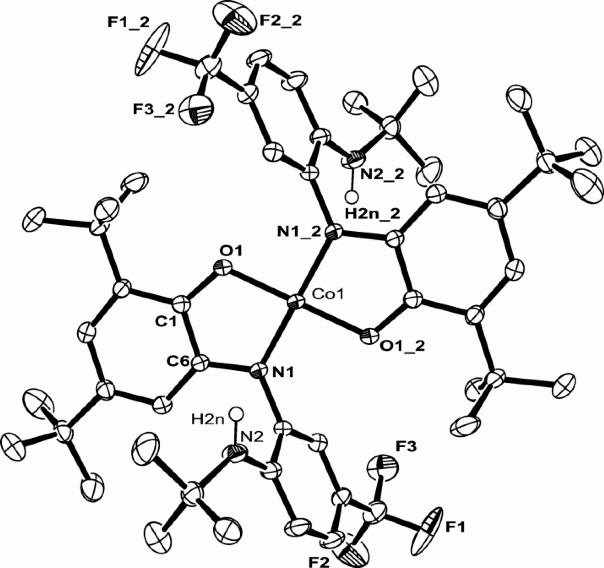
Ni(bTzP)Cl2(MeCN), (shown below) can be synthesized by refluxing NiCl2·THF1.5and bTzP ligand in MeCN. The product was crystallized by slow evaporation in MeCN to yield bright orange crystals.
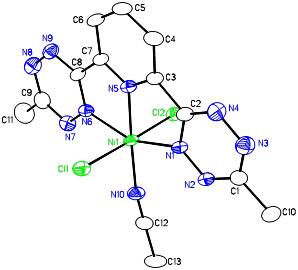
These two Ni species can then act as a platform from which we will make, by reaction with reducing agents, unsaturated Ni complexes with electrons stored in the bTzP ligand.