Biography
Keith Searles was born and raised in Richmond, VA. In the city if Richmond he attended Atlee High School for three years and graduated from Hermitage High School in the spring of 2005. Keith made the decision to remain close to his hometown by attending Randolph-Macon College, located approximately 15 miles north of Richmond, VA. At Randolph-Macon College he obtained a B.S. in chemistry while researching the theory of “ion exclusion” in thin ice films under the guidance of Dr. Rebecca Michelsen. After graduation from Randolph-Macon College in the spring of 2009, Keith became an employee of the college while working as a research assistant for Dr. Serge Schreiner. Under the advisement of Dr. Schreiner, Keith’s research focused on the synthesis of rhodium and iridium complexes, stabilized by bis(dicyclohexylphosphino) methane, for use as potential catalysts. In the fall of 2010, Keith began pursuing a doctorate in chemistry at Indiana University. He continues his research focus in organometallic chemistry under the advisement of Professor Daniel J. Mindiola and Professor Kenneth G. Caulton.
Research Projects
The Caulton group is currently interested in the design and application of redox noninnocent ligands. Not only are we interested in the synthesis and full characterization of these metal complexes, but we also want to investigate the application of these complexes in catalysis. We envision using a single ligand, which we can electronically tune to favor the reduction or oxidation of various substrates.
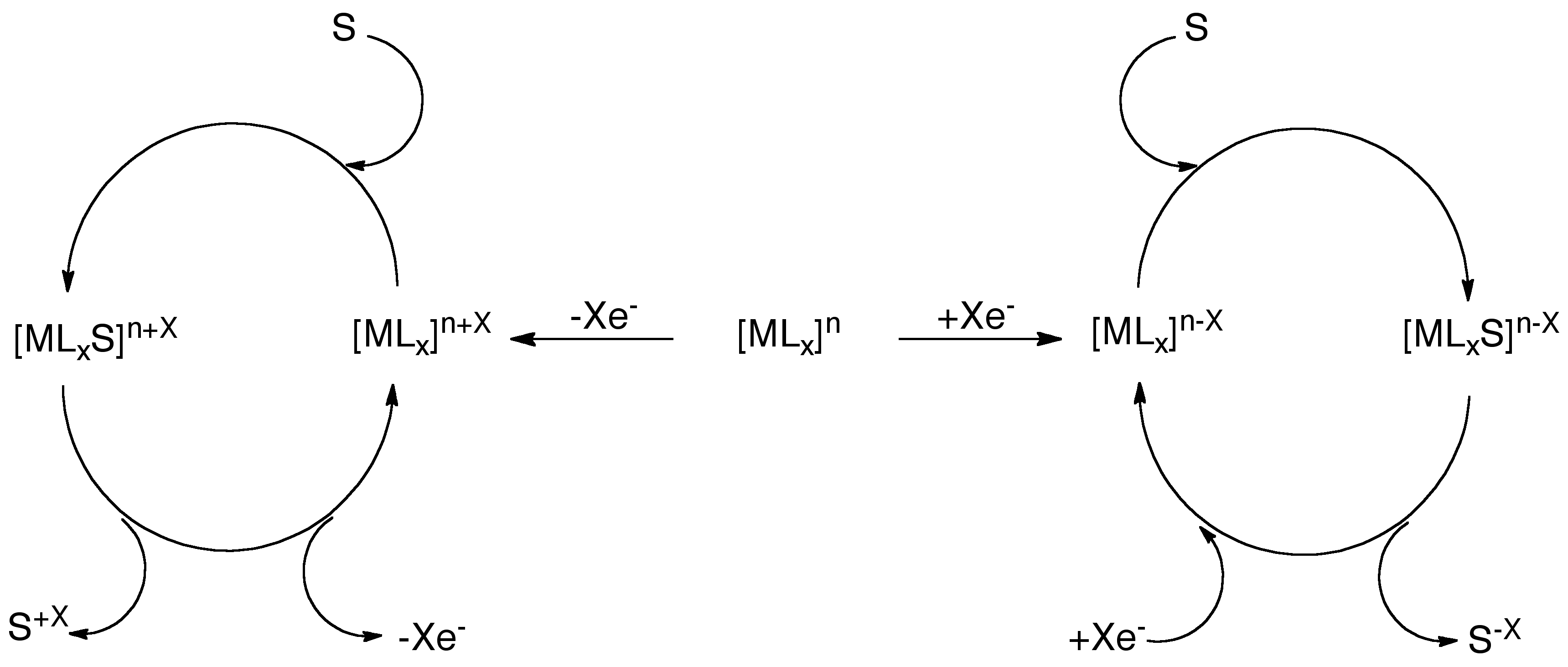
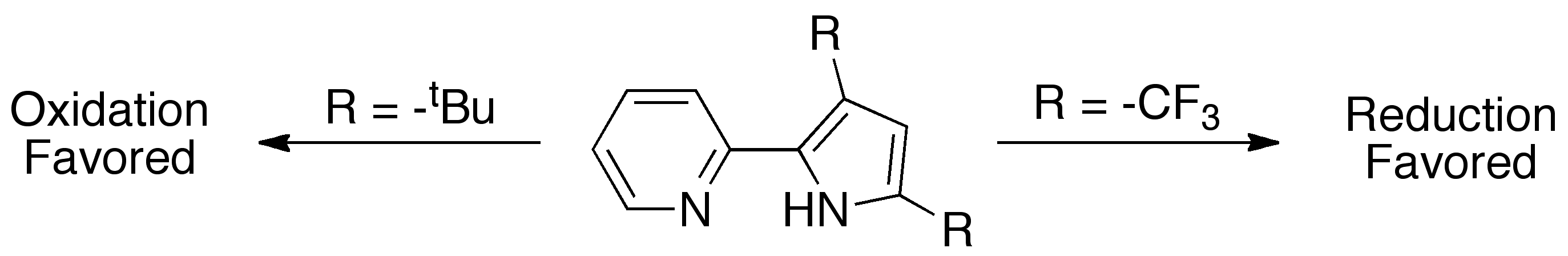
The ligand class we are employing is pyridylpyrrolide, which has a high degree of electronic tunability. The synthesis of the ligand allows for the substitution of the 3,5-positions on the pyrrolide with either electron withdrawing trifluoromethyl or electron donating tert-butyl substituents, which have a direct effect on the redox behavior of the ligand itself. The electron donating tert-butyl substituents favor the oxidation of the pyrrolide π-system, while the low-lying π* of the pyridyl in combination with elctron withdrawing trifluoromethyl groups favor reduction. This allows for tunable redox behavior in pyridylpyrrolide complexes, where the redox processes do not have to be 100% metal centric. Thus, pyridylpyrrolide can serve as either an electron reservoir or electron hole to aid the metal center.
I am currently investigating the redox properties and reactivity of Co(L)2complexes. Having synthesized two varieties, one with tert-butyl groups and the other with trifluoromethyl groups, I am able to directly compare the reactivity and redox properties of the two complexes, which possess drastically different electronic properties.
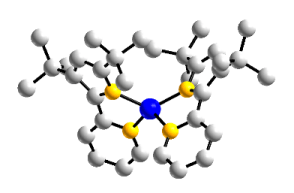
R = –tBu
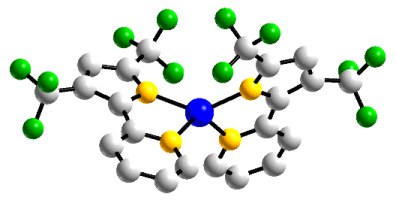
R = -CF3
After fully characterizing these complexes, my future work will focus on fine-tuning the electronic properties of these complexes and using redox chemistry to induce further reactivity in catalysis.

