Biography
Originally, I am from the ‘Land of Enchantment’, otherwise known as Albuquerque, New Mexico. I graduated from Butler University in Indianapolis, Indiana with my B.S. in chemistry. During my time at Butler, I was graced the opportunity to synthesize and characterize a variety of seven coordinate Group VI transition metal complexes. I performed this work under the guidance of Dr. Stacy O’Reilly, who was one of the most important influences that drove me towards inorganic chemistry during my undergraduate career. I was afforded the opportunity during a summer research project to characterize a variety of these tungsten and molybdenum transition metal complexes, which drove me towards a desire to learn more about the capabilities these transition metals contain. This passion is what drove me into Ken Caulton’s group.
Research Project
N-heterocyclic carbenes (NHC) are ligands that have been known for quite awhile to be very strong σ-donors, allowing them to act as robust ligands that form strong bonds to metal centers, with little tendency for dissociation. NHCs can carry sterically hindering substituents as well, which prevents ligand dimerization, leaving open metal coordinate sites for substrate binding. . NHCs have since been shown to have inherent properties that are analogous to ligands much like trialkylphosphines, allowing for their use in areas such as metal-based inorganic and organometallic coordination chemistry, as well as homogeneous catalysis. One such example of this catalysis is shown below, where an NHC ligand is a key ligand within the Suzuki coupling of aryl chlorides.

New variations of these ligands exploits their extreme versatility in both flexibility of the ligand, as well as their structural and electronic properties.
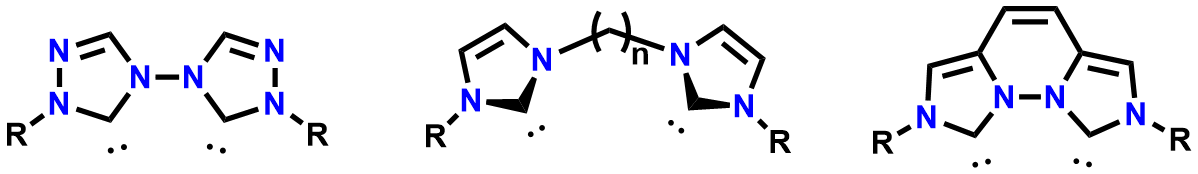
Figure 1. Structures of NHCs that demonstrate both flexibility and rigidity ligand can possess, along with the various structural and electronic properties ligand can possess.
I hypothesize that these ligands have the possibility of providing a metal center the stabilization necessary for undergoing a variety of chemical transformations which might not normally be accessible with a weaker σ-donating ligand. I propose that a ligand with two carbene sites and a rigid backbone would essentially force the ligand to bind to a metal in a planar fashion. This is favorable binding for certain group 10 metals, such as Ni2+, binding the metal in a pseudo square planar manner. This allows for two open binding sites on the opposite open face of the metal. Tuning of the steric and electronic properties on this bis(NHC) ligand opens the possibility of selectively tuning the reactivity of the chosen metal center towards a variety of substrates.
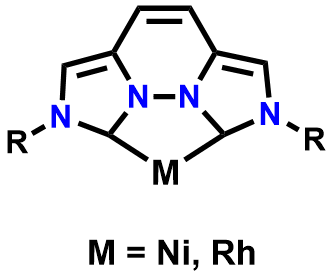
Figure 2. Structure utilizing the Bis(NHC) ligand for the bidentate binding properties to both group 9 and 10 metals
As shown in the above depiction, there is potential for unfavorable strain upon binding of the rigid NHC with one metal. However, it has been proven that the bidentate binding mode of the NHC ligand will be comparable to that of a 1,10 phenanthroline ligand, with respect to characteristics such as bite angle and bond distance.
I also hypothesize that if it is possible to bind one NHC ligand to one metal, then it is theoretically plausible to have the NHC bind to two metals, leaving open binding sites on two metals instead of one. This would be ideal since two metals could provide twice the activation power to a bound substrate, allowing for accessibility to previously unattainable intermediates due to labile reactivity. Shown below is one proposed situation for this dinuclear metal-metal system, in which the metal complex is bound to a nitrate substrate, allowing for open reactivity on the nitrate via the metal center.


