Biography
Nobuyuki Komine was born and grew up in Tokyo, Japan. He received his Ph.D. in March 1998 under the supervision of Professor Takeshi Nakai and Dr. Katsuhiko Tomooka at the Tokyo Institute of Technology. He joined the S. Komiya and M. Hirano research groups in Tokyo University of Agriculture and Technology (TUAT) as a Research Associate in April 1998. He became an Assistant Professor of TUAT in 2007. In April 2011, he joined the Caulton and Mindiola groups as a Postdoctoral Fellow working on C-H and C-C bond insertion chemistry involving coinage metal catalysts.
Research Projects
Alkanes are compounds of carbon and hydrogen atoms constructed by only C-C and C-H single bonds. The simplest and most abundant is methane, the primary constituent of natural gas. The use of alkanes as environmentally benign feedstock for clean-burning fuels and a host of petrochemicals is a high impact goal. The development of a new catalyst for selective, direct alkane functionalization could lead to a new paradigm in petrochemical technology that is environmentally cleaner and economically superior. In our research group, the reaction where insertion of the carbene derived from N2C(H)(CO2Et) into C-H bonds of sp3 carbons is the focus of my work. The catalyst for this reaction is a trimeric Ag(I) complex with a cis-bidentate monoanionic nitrogen ligand, 2-pyridylpyrrolide. My research is the development of new homogeneous catalysts for alkane functionalization by this catalyst system.
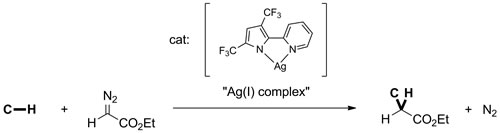
Scope and limitation
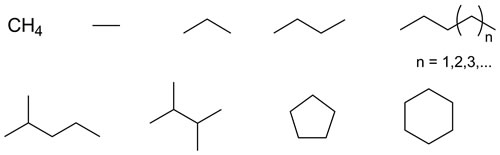
Selectivity
![]()
Mechanistic Investigation of Alkane Functionalization
Our catalyst also inserts the carbene fragment into the C/C bond of benzene to give a cycloheptatriene, which is regarded as extremely surprising in terms of breaking such a strong, resonance-stabilized C/C bond, that in the arene. DFT calculations show that this reaction is thermodynamically favored, but less so than carbene insertion into the arene C-H bond; thus, the catalyst accomplishes non-thermodynamic selectivity; here, mechanism is more important than thermodynamics.
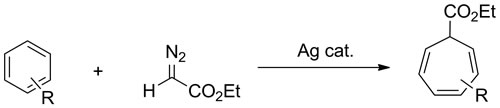
My results may provide a new molecular alkane functionalization method that is environmentally cleaner, economically superior and allows the large reserves of untapped remotely located natural gas to be better used as primary feed stocks for fuels and chemicals.