Biography
I grew up in Seoul, Korea and moved to Richmond, Virginia when I was fifteen. I graduated with a BS in chemistry from Randolph Macon College. While at RMC, I was given opportunities to have three years of undergraduate research experience in Serge Schreiner’s organometallics research group including two summers of research. My undergraduate research focused on small molecules activation by the PNP pincer ligated transition metal complexes (groups 9 and 10 metals!) and CO2 reduction with the activated complexes. Outside of chemistry, I love doing music: listening, playing (I’ve played violin and piano since I was six). Other than that I like taking a nap and just slacking around a lot.
Research Projects
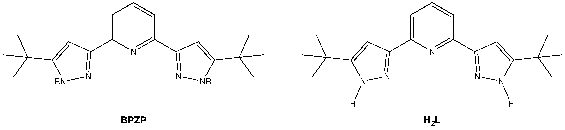
In my C500 project, reactivity of two low valent chromium (Cr(II) and Cr(III)) compounds stabilized by bis(pyrazolyl)pyridine (BPZP) towards nitrate activation will be explored with the goal of contributing to design of metal complexes that are capable of proton coupled electron transfer (PCET) to accomplish reductive deoxygenation of the oxoanions such as NO2– and NO3–.
NO3– + 2H+ + 2e– → NO2– + H2O
NO2– + 2H+ + 2e– → NO– + H2O
The redox active ligand, BPZP, used in this study is with R groups being proton (H2L) which may play a key role for the nitrate reduction. For instance, protons are bonded to N of the pyridine ring outwardly, thus, once NO3– is coordinated to the metal center, the prolific proton can possibly interact with the closest O atom of the nitrate by hydrogen bonding followed by N-O bond cleavage (*).
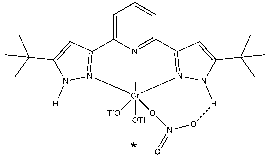
Prior to the activation of NO3–, as the previous studies in the group showed that the metal-ligand chlorides precursors have low solubility, I am currently working towards chloride substitution using TMS-triflate to improve the solubility.
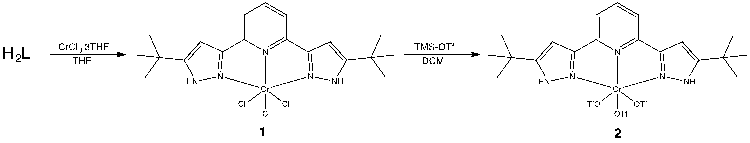
Once compound 2 is prepared and fully characterized, nitrate coordination and reduction will be thoroughly studied. An analogous study with Cr(II) will be carried out.

