Biography
I am originally from the great state of Maine, born and raised in the small city of Waterville. I graduated from Williams College, seated in the beautiful Berkshires of western Massachusetts, receiving a Bachelor of Arts with Honors in Chemistry in 2015. During my time at Williams, I did undergraduate research under the mentorship of Chris Goh working towards the development of aluminum-based ring-opening polymerization catalysts for the synthesis of polylactide, a biodegradable polymer derived from a renewable source. After taking a gap year post-graduation, I came to Indiana University with a passion for catalysis and aspirations to contribute towards a greener future. Naturally, this lead me to the Caulton group. Outside of chemistry, my interests include spending time in the great outdoors, live music, and sports (particularly football and basketball).
Research Projects
The reduction and recycling of environmentally-detrimental small molecule pollutants is a critical, yet unresolved, challenge faced by the global community. Processes to convert these pollutants into value-added products typically require the input of electrons and this has generated wide interest in transition metal catalysts containing redox-active ligands able to store and transport electrons.
Quinones and tetrazines are well-known for their redox capabilities and represent an unutilized class of ligands applicable to these types of molecular transformations. Our work seeks to link the fundamentally interconnected fields of heterogeneous and homogeneous catalysis through the rational design of ligands that promote self-assembly with atomic metals to form one and two-dimensional on-surface coordination polymers that may store and transport electrons to targeted substrates.

Our current work primarily focused on tetra-aza-anthraquinone ligands which have to potential to be reduced, by deposited elemental metal, by up to four electrons. Deposition to a surface followed by addition of metal results in highly disordered chains which we hypothesize is the result of numerous coordination geometries being allowed by the ligands four equivalent binding sites. This has lead us to more carefully design ligands that have greater control of coordination environment and directionality.
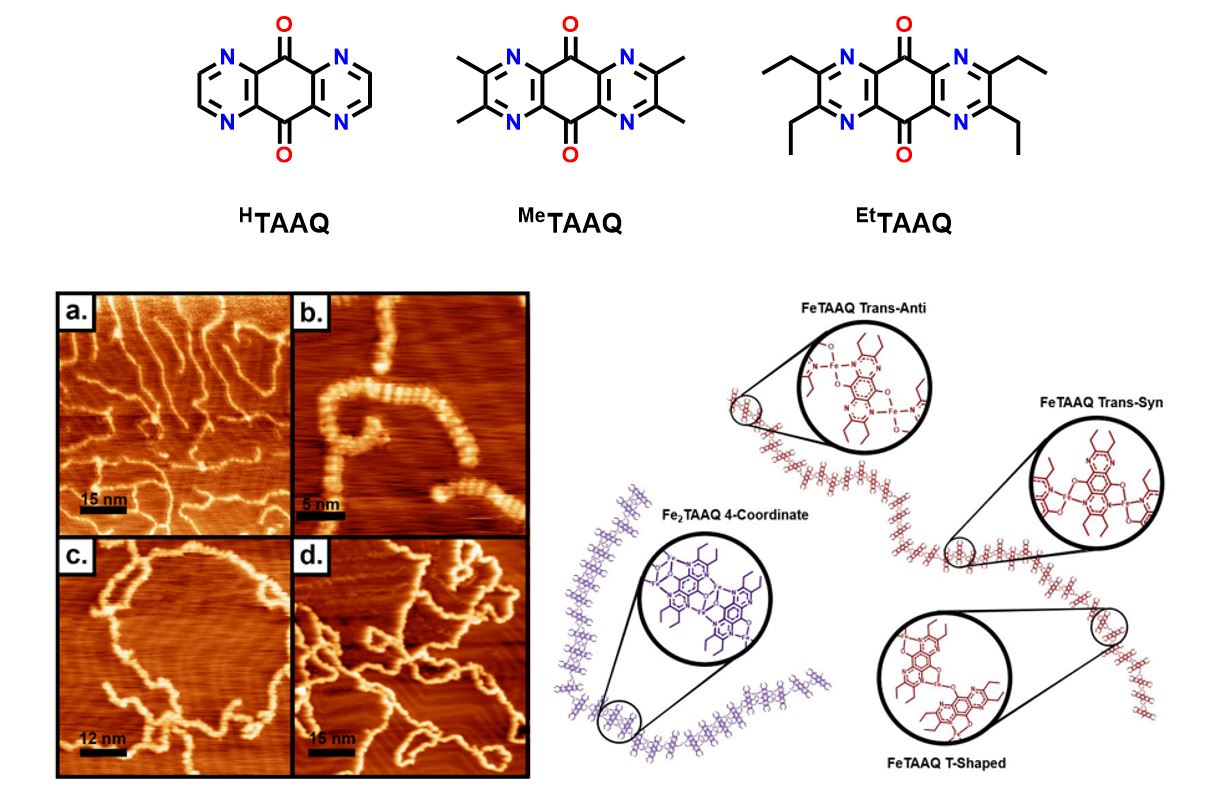
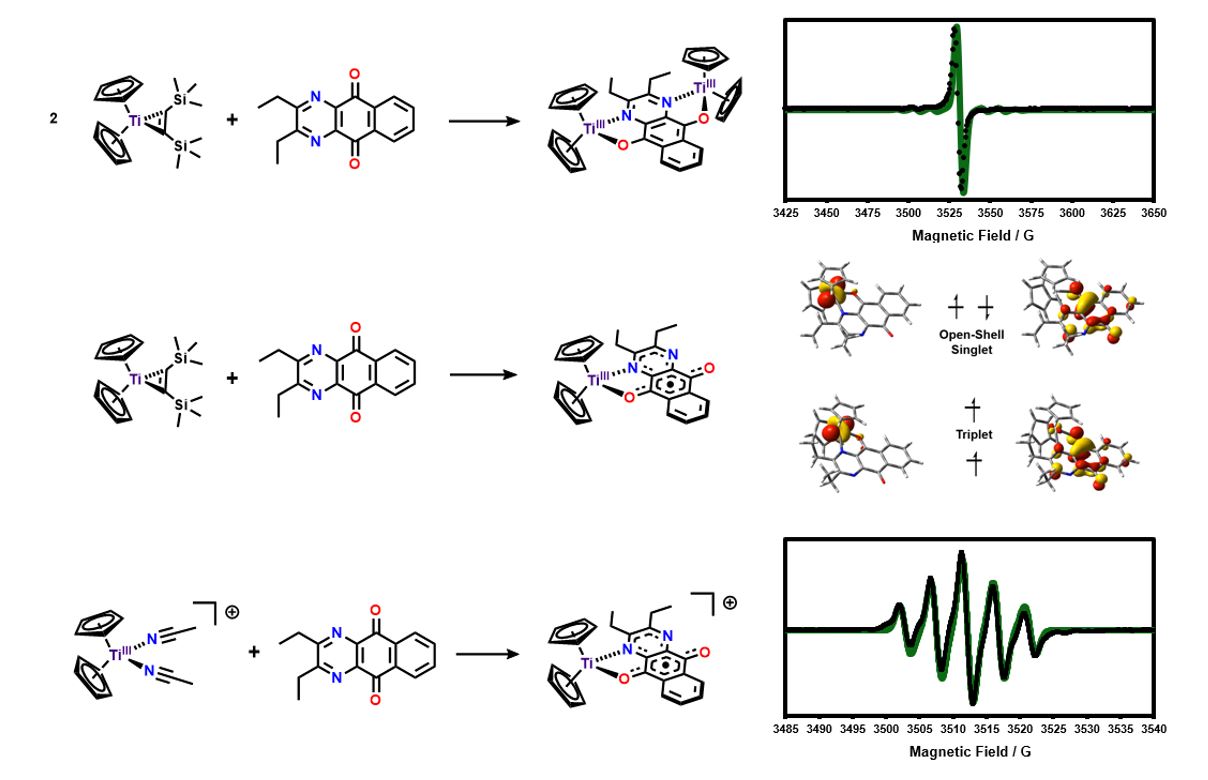
My primary role as the molecular side of our collaboration is to design and synthesize new ligands to be used on the surface (see below). Additionally, I synthesize small-molecule analogues, e.g. for Ti, below, of our surface species so that we can better understand the redox and spin localization properties of our systems using traditional molecular characterization techniques.