Biography
Immanuel Reim is a visiting scholar from Stuttgart, Germany. He was given the opportunity from the chemistry department of Indiana University Bloomington to join any research group for the upcoming year. Within this program he choose to work in Caulton’s Lab. The program encourages not only the scientific exchange but also cultural exchange to show the differences and similarities between Stuttgart/Germany and Bloomington/USA.
He grew up in Backnang, Germany and finished school at the Max-Born-Gymnasium Backnang in 2011 where he received the GdCH-award. He started his chemical education at the University of Stuttgart in 2011. During his second year he worked as a research assistant at the Fraunhofer Institute IPA in Stuttgart to investigate the dependence of the hardening process of polymers on the initiator concentration. He graduated in 2014 working for Dr. Dietrich Gudat. In the following summer, he joined Dr. Francesca Kerton’s group at the Memorial University of Newfoundland, St. John’s Canada, working on the catalytic ring-opening polymerization of epoxides due to the exchange program RISE organized by the DAAD (German academic exchange service). Currently, he is studying towards his Master degree at the University of Stuttgart.
Research Projects
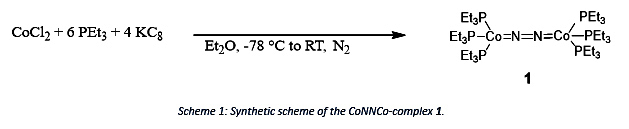
Immanuel Reim’s research goal is to investigate and characterize the recently found formally zero-valent CoNNCo-complex by Brian Cook towards its reactivity and its usage as reducing agent as each Co can function as a one or two electron reductant.
Therefore, different oxidants will be used to utilize its property. Below, several oxidants are shown which can work as two electron acceptor.
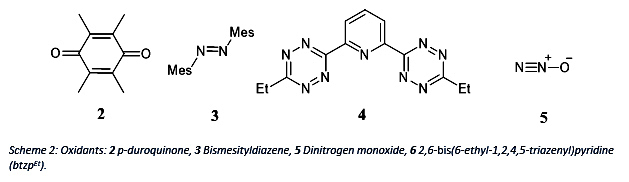
The redox potential of btzpEt 4 is investigated by my coworker Nicholas Maciulis. Therefore, both projects will benefit from gained results of this reaction.
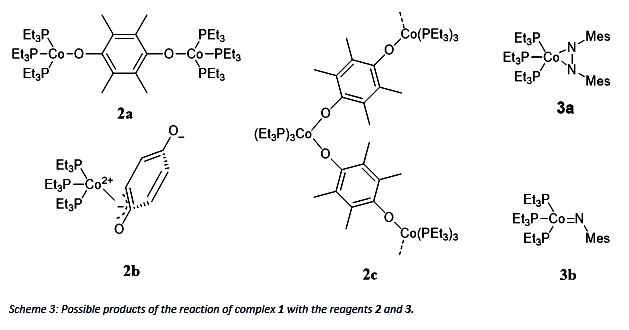
The reagent 2 can either replace the N2 in the original complex 1 and oxidize each Co from 0 to +1 to gain compound 2a or oxidize Co by 2 and form a coordinative single complex 2b or a coordinative polymer 2c. It has to be mentioned that the stoichiometry for product 2a is 1:1 (complex 1: duroquinone) whereas for the product 2b and 2c a stoichiometry of 1:2 is necessary. In comparison reagent 3 will oxidize Co by 2 but can form either a side-on adduct 3a or a terminal 3b product by breaking the NN double bond.
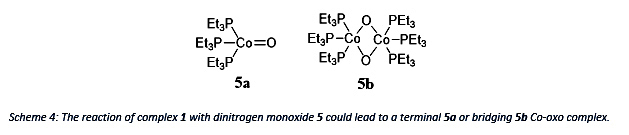
Using Dinitrogen monoxide as an oxidant could lead to the terminal Co-oxo complex 5a which would be interesting since it would challenge theory of the “Oxo-Wall”. But according to theory, the product 5b with two bridging oxygens is more likely.
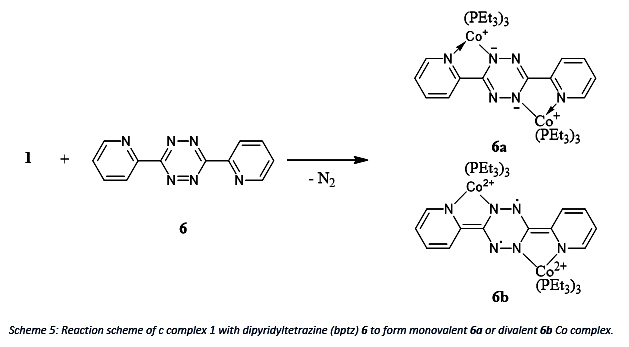
To test its ability further, the reaction of complex 1 with dipyridyltetrazine (bptz) 6 will be investigated since bptz can be reduced by either 2 or 4 electrons which results in a monovalent 6a or divalent 6b Co. The reduction of bptz can be seen in the change of the 1H NMR chemical shifts. Note, that the divalent Co complex is a diradical species.