Biography
Skye Fortier is a native of the Southwest, born and raised in the far West Texas town of El Paso, which is directly located on the US/Mexico border. As an undergraduate, Skye attended the University of Texas at El Paso (UTEP) where he received a MARC (Minority Access to Research Careers) scholarship. As a MARC scholar, Skye worked under the direction of Prof. Keith Pannell investigating the photochemically induced formation of carbon-silicon bonds utilizing ‘Fp’ precursors (Fp = CpFe(CO)2). After graduating in 2005, Skye worked for one year as a high school science teacher at Irvin High School in El Paso teaching freshmen and junior classes.In 2006, Skye entered the graduate program at the University of California, Santa Barbara (UCSB). At UCSB, Skye worked in Prof. Trevor Hayton’s laboratory investigating the organometallic chemistry of uranium. In particular, his studies in the Hayton laboratory focused on the synthesis of high-valent homoleptic molecules and uranium complexes featuring metal-ligand multiple bonds. Graduating from UCSB with his Ph.D. in Fall 2011, Skye traded in the West Coast for the Midwest in order to work as a postdoctoral researcher under the joint supervision of Profs. Mindiola and Caulton. At IU, he is investigating the synthesis, reactivity, and redox chemistry of metal complexes supported by non-innocent, redox-active frameworks. Skye is a recipient of an NSF American Competitiveness in Chemistry Postdoctoral Fellowship.
Research Projects
My research is broadly targeted towards investigating the synthesis, reactivity, and redox chemistry of low-valent, first-row transition metal complexes supported by non-innocent, redox-active frameworks. The ultimate goal of this work is to develop electron-rich complexes, incorporating inexpensive earth abundant metals, for use in catalysis and group transfer chemistry.
Currently, my project is centered on examining the use of a brand new redox-active ligand platform, namely the indigo derivedN,N’-diaryldiimines (best known as ‘Nindigo’) recently developed by the Hicks’ Group at the University of Victoria. Nindigo is novel in the sense that it is binucleating, consisting of two nacnac-type fragments. Moreover, Nindigo is a modular system; the sterics of the imine substituents can be tuned depending on the aniline used during the condensation reaction with indigo. Finally, Nindigo is highly conjugated which is clearly evinced by itsintensedeep purple color – staining all that it touches!
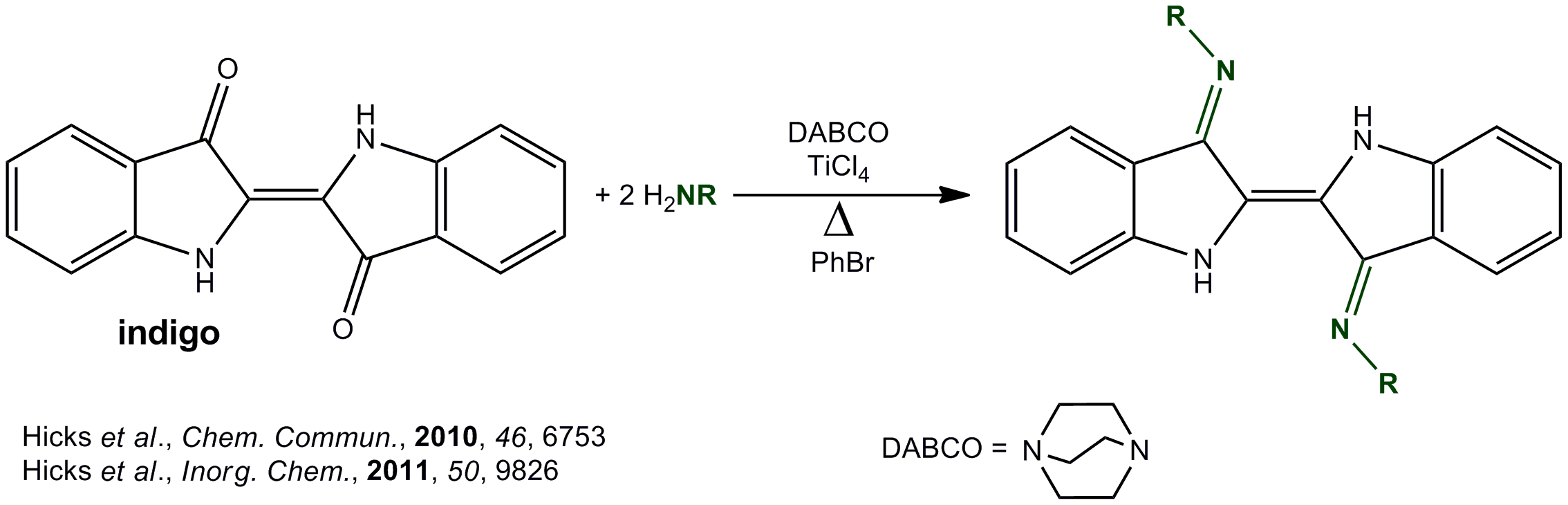
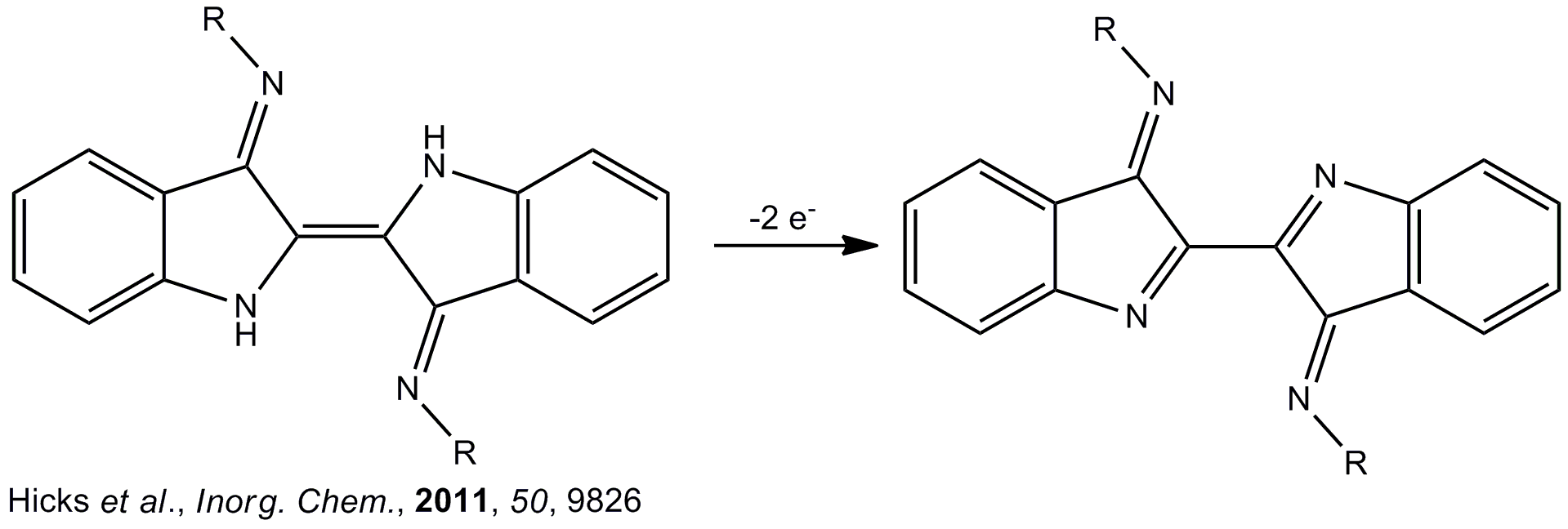
It has been established that free Nindigo can readily undergo a two-electron oxidation process to generate neutral, stable dehydroNindigo species. In principle, this means that Nindigo possesses the capacity to act as an electronic reservoir capable of releasing electrons. When complexed to a metal, it is anticipated that a synergetic metal-ligand effect will occur affording multi-electron reactivity that extends beyond the capabilities of the metal center itself.
My research has revealed that Nindigo can be readily deprotonated using silylamide bases. For instance, treatment of H2dmp2Nindigo (dmp = 2,6-dimethylphenyl) with 2 equivalents of Li[N(SiMe3)2] readily affords dmp2Nindigo[Li(THF)2]2in good yields.
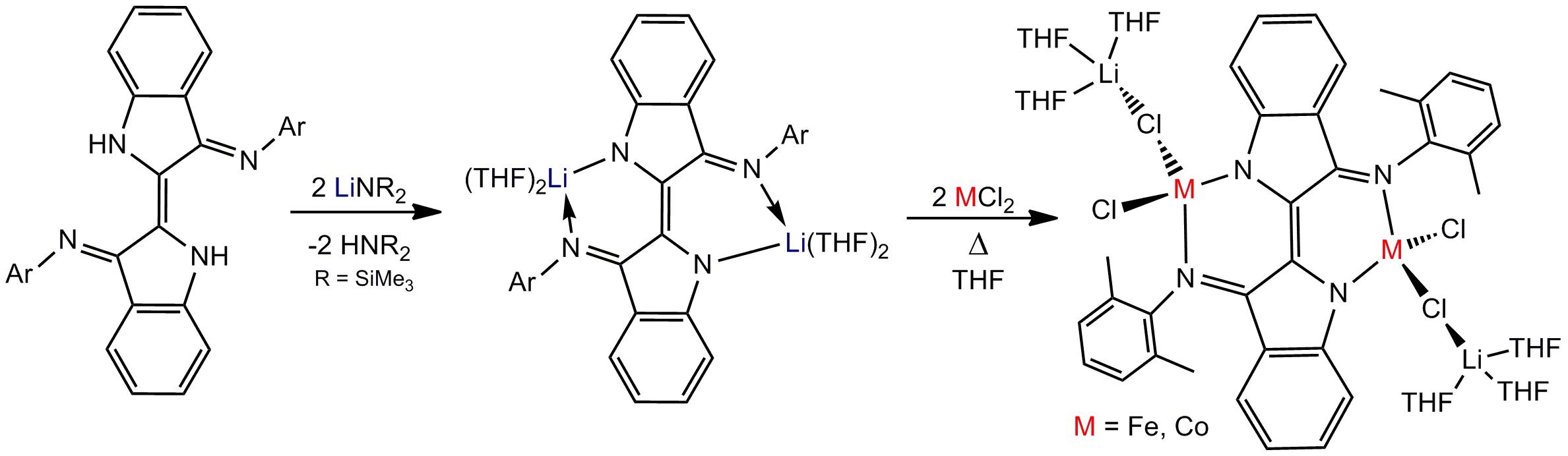
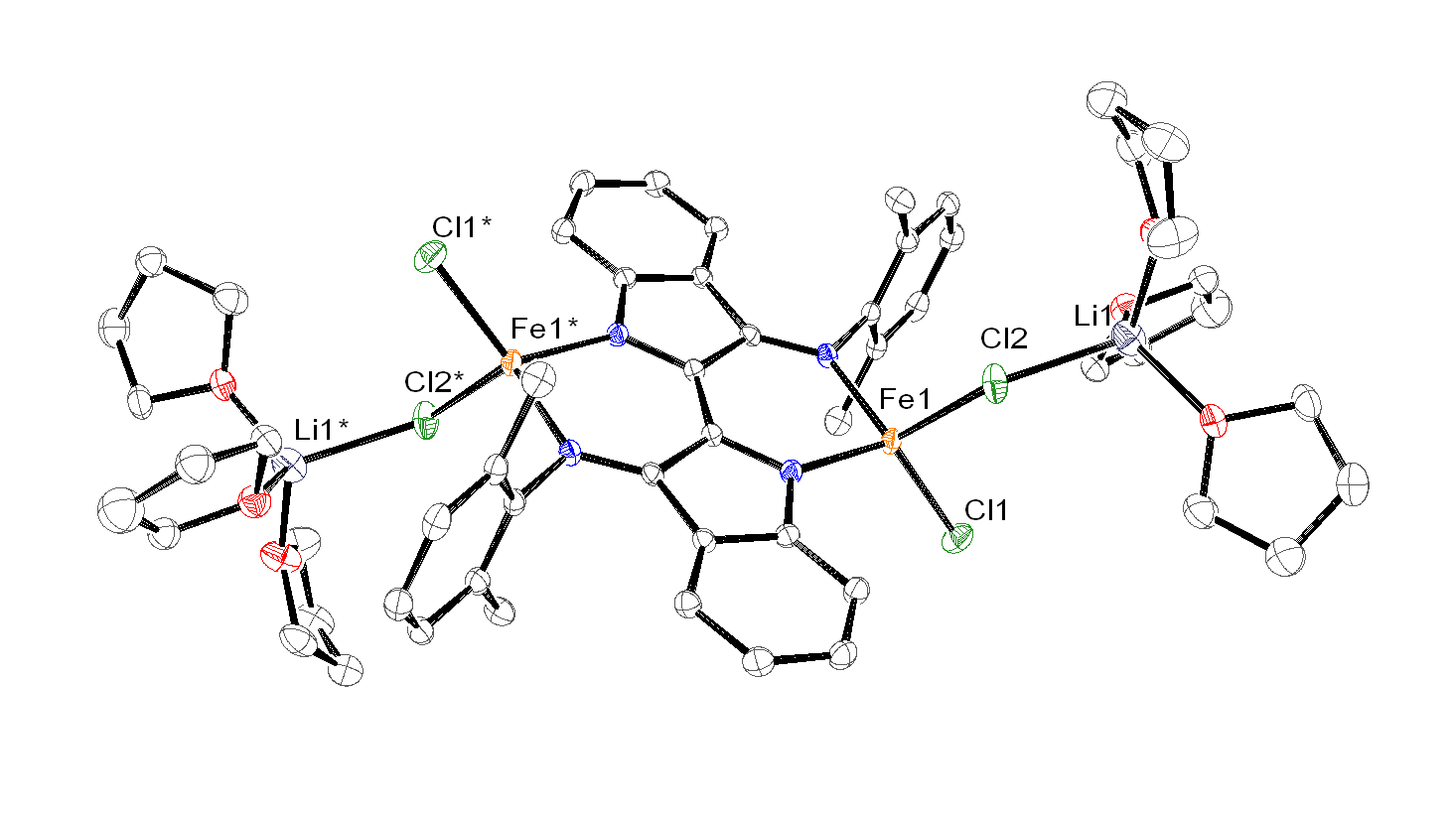
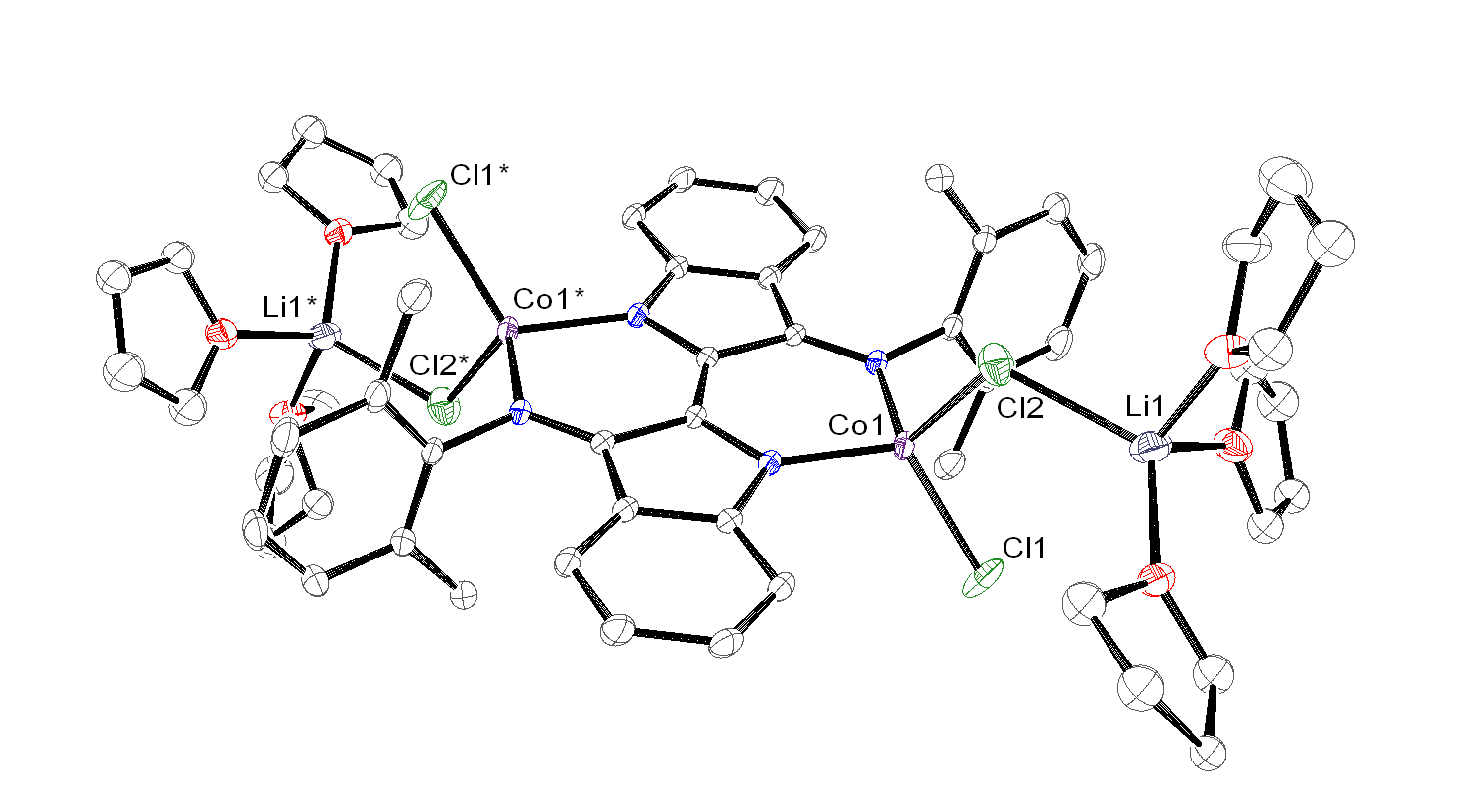
Reaction of dmp2Nindigo[Li(THF)2]2with metal halides, namely CoCl2and FeCl2, affords the tetrahedral metal ‘-ate’ complexes dmp2Nindigo[MCl{Li(THF)3Cl}]2(M = Co, Fe).
Additionally, reaction of H2dipp2Nindigo (dipp = 2,6-diisopropylphenyl) with Co(NR2)2(R = SiMe3) yields the tetrahedral Co(II) complex dipp2Nindigo[Co(NR2)(THF)]2. In a similar fashion, I intend to use M(NR2)2with Nindigo to generate other Nindigo[M(NR2)]2species.
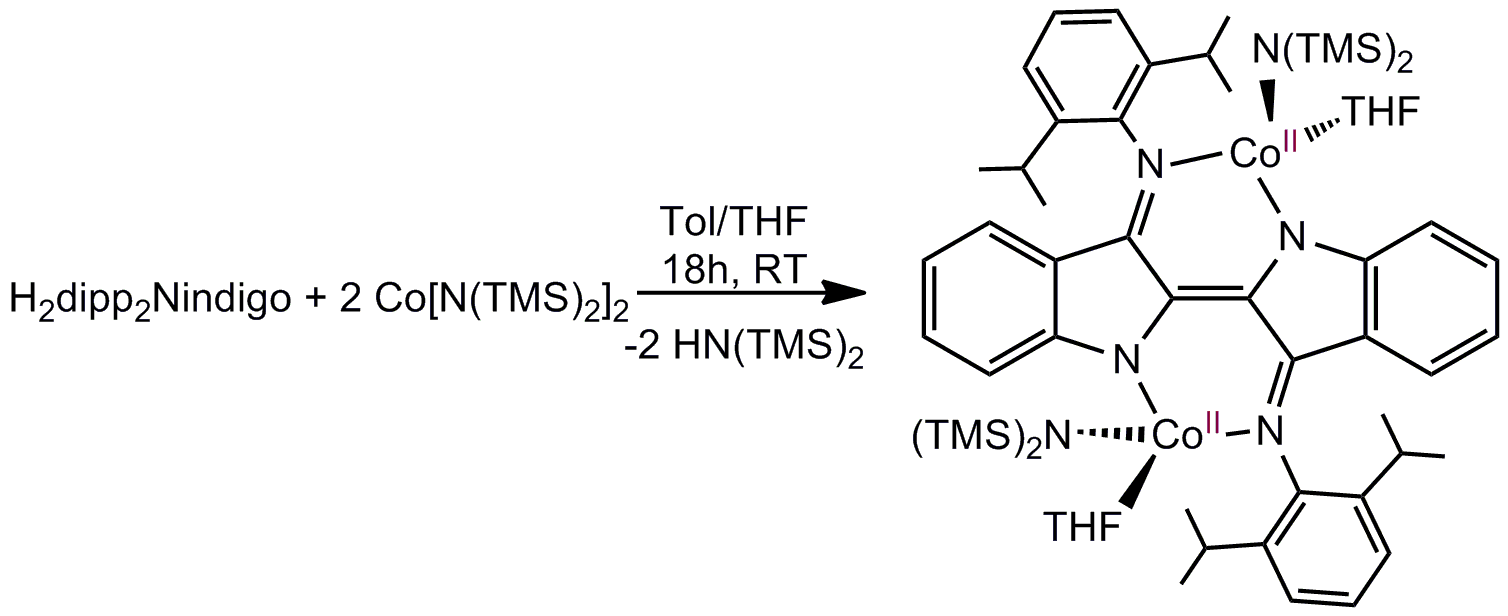
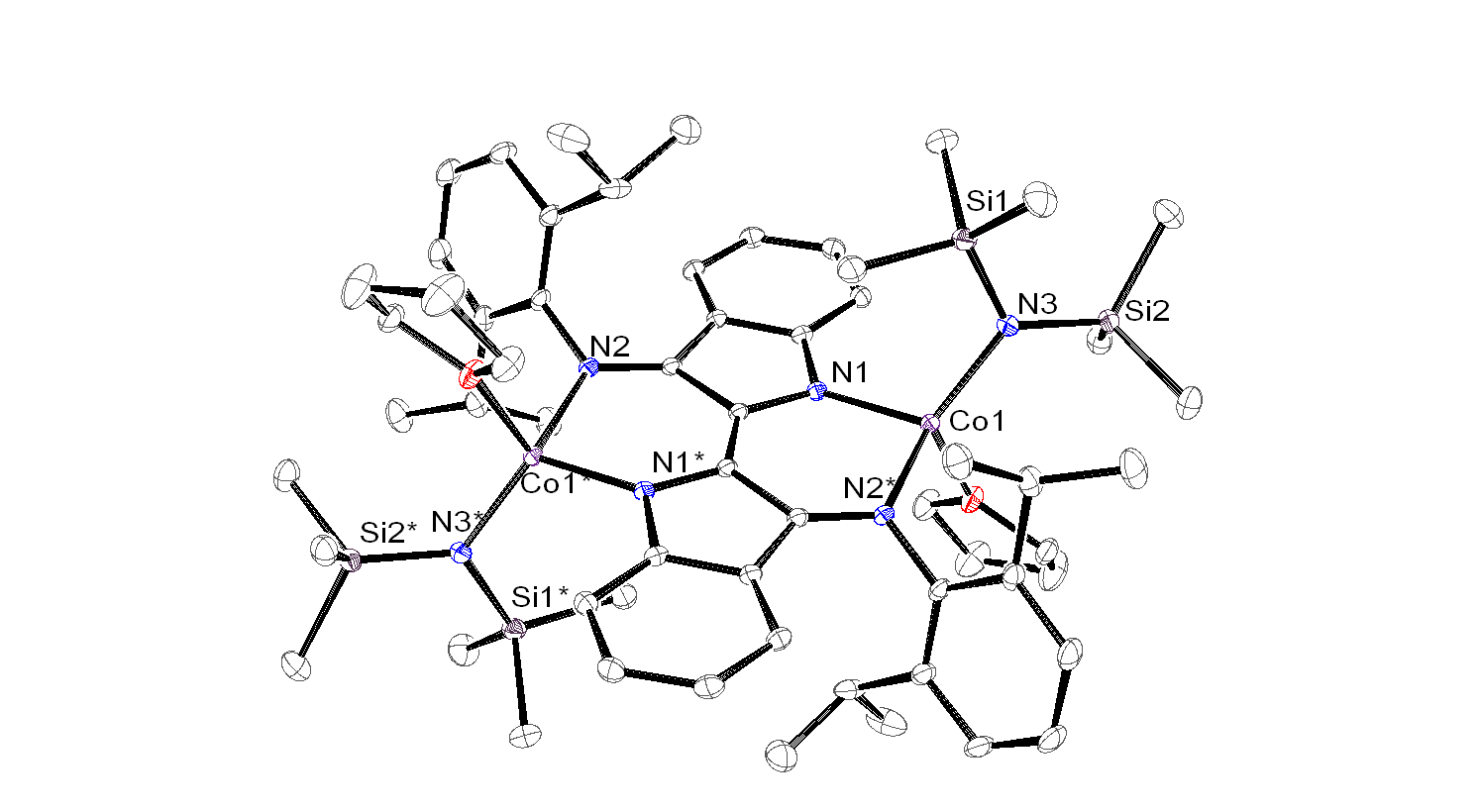
These complexes will serve as the platform for my investigation into the reactivity of first-row transition metal Nindigo systems.
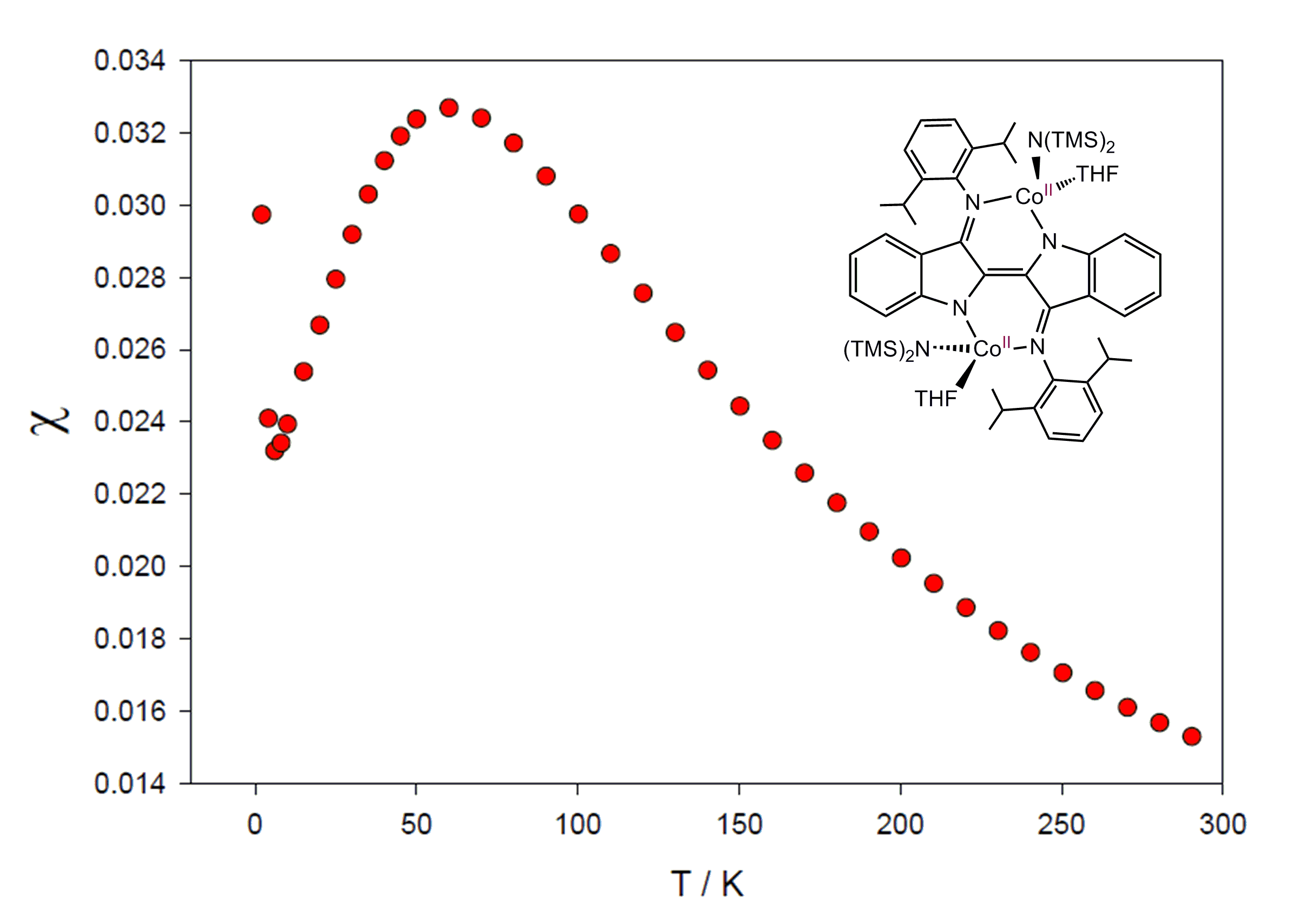
Finally, it should be noted that given Nindigo is both highly conjugated and binucleating, the potential for intramolecular metal-metal communication exists which could allow for interesting magnetic and electronic properties. Indeed, I have found that analysis of paramagnetic dipp2Nindigo[Co(NR2)(THF)]2by SQUID magnetometry reveals antiferromagnetic coupling between the Co(II) centers at low temperatures.