Biography
I was raised at the base of the Smoky Mountains in Sevierville, Tennessee, before attending Belmont University in Nashville, Tennessee to earn a B.S. in chemistry. While at Belmont, I had the opportunity to synthesize and characterize Schiff-base nickel(II) complexes under the mentorship of Dr. Justin Stace. I truly knew that graduate school was the place for me after an REU at the University of Cincinnati, where I worked in Dr. William Connick’s lab. When I’m not in the lab, I enjoy spending time hiking, running, playing piano, and watching sports. Indiana University offers a welcoming environment, excellent facilities, and a beautiful location to call home. I am motivated by a desire to engage in meaningful research at the forefront of the field, and the Caulton group pushes the boundaries of inorganic research involving CO2, NO3-1, and NO2-1 reduction – important research that will help me achieve my goal of making a difference through chemistry.
Research Projects
A pre-reduced pincer ligand, H4btzp, can be easily synthesized and has the ability to store 4 protons and 4 electrons, as presented in the half reaction in Scheme 1.
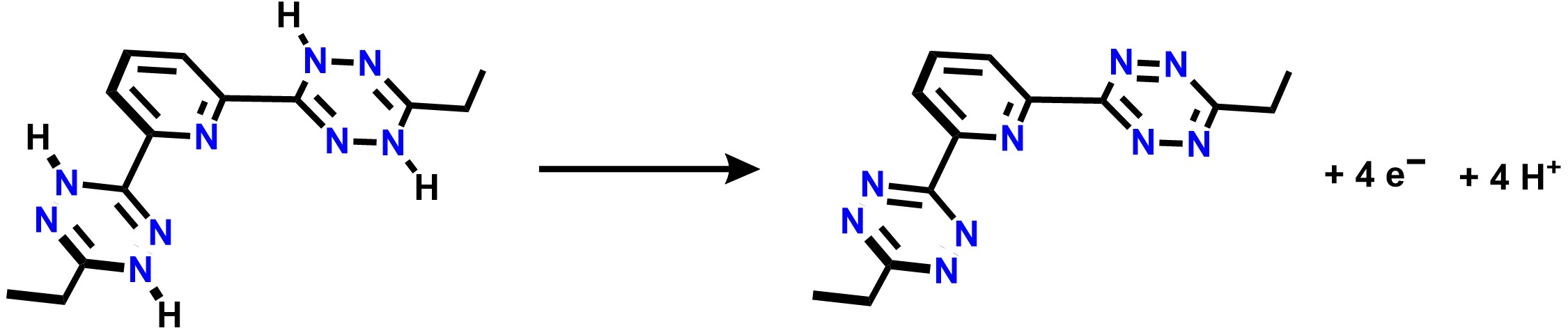
Scheme 1. Half reaction for the oxidation of H4btzp to btzp
We envision that H4btzp is an attractive ligand for nitrogen oxyanion reduction as it has the necessary electrons to reduce high oxidation state nitrogen oxyanions, such as nitrate and nitrite, while also having protons to sequester the O2- released from N-O bond cleavage (Scheme 2).
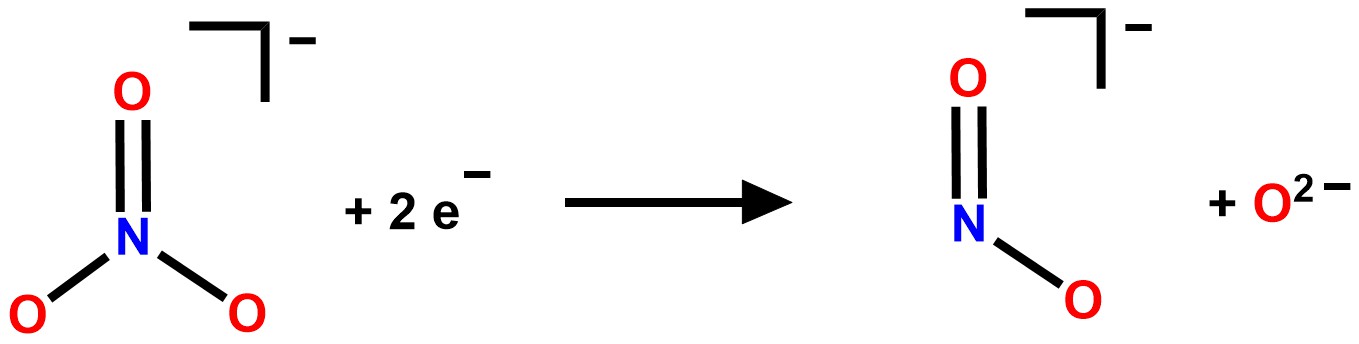
Scheme 2. Half reaction for the two electron reduction of nitrate to nitrite
With redox chemistry intended to happen at the ligand, we chose the redox-inert zinc as our starting metal. H4btzpZnCl2 can be synthesized from H4btzp and ZnCl2, and salt metathesis with two equivalents of AgNO3 yields H4btzpZn(NO3)2. When two equivalents of AgNO2 are used instead, there is complete oxidation of H4btzp to btzp, with two equivalents of H2O formed, as monitored by 1H NMR. The oxygens in H2O come from nitrite, indicating that reduction has occurred. We can confirm the presence of NO gas from this reaction through the use of an NO scavenger, paramagnetic Cr(N(SiMe3)2)3, which readily reacts with NO gas to give diamagnetic Cr(NO)(N(SiMe3)2)3. Therefore, H4btzp when bound to zinc selectively reduces nitrite by one electron to yield NO gas, and full redox balance is shown in Scheme 3.
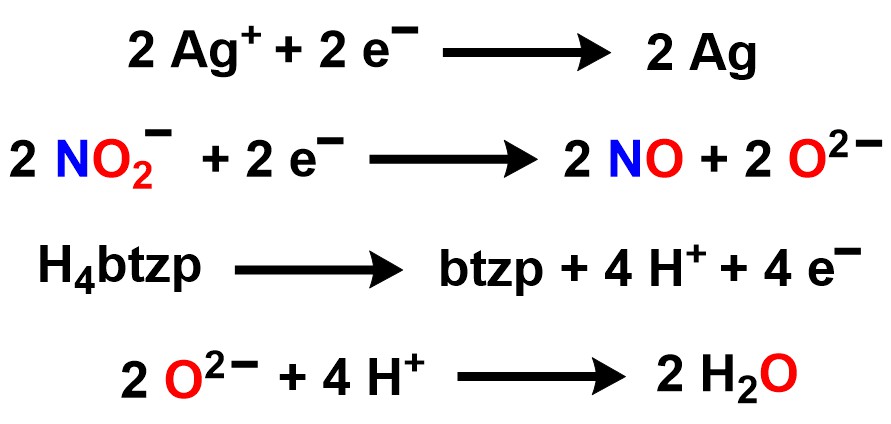
Scheme 3. Redox balance for the reaction of H4btzpZnCl2 with two equivalents of AgNO2
In an effort to capture reduced nitrogen containing species, we moved from zinc to iron. H4btzpFeCl2(MeCN) can be synthesized from FeCl2 and H4btzp in CH3CN. When H4btzpFeCl2(MeCN) is reacted with two equivalents of AgNO3, salt metathesis proceeds as expected to give H4btzpFe(NO3)2. However, when H4btzpFeCl2(MeCN) is reacted with two equivalents of NaNO2, there is complete oxidation of the pincer ligand and formation of H2O observed by 1H NMR. The IR spectrum of the reaction mixture indicates the formation of a di-nitrosyl iron complex (DNIC), currently under study. Because our nitrite delivery source in this case cannot act as an oxidant, the formation of this DNIC indicates that all four protons and four electrons stored in H4btzp are delivered to substrate.

