Biography
Brian Jeffrey Cook was born in Trenton, New Jersey in 1989 and grew up in the neighboring suburb of Lawrenceville, NJ. An interest in chemistry grew out of proficiency in high school and led to Brian double majoring in Biochemistry and Chemistry/ACS Certification track at Seton Hall University (South Orange, NJ campus). While at Seton Hall, Brian received numerous commendations, including the Undergraduate Organic Chemistry Award from the American Chemical Society Divisions of Polymer Chemistry and Polymer and Materials Science, and the New Jersey Institute of Chemists award. At Seton Hall, Brian was involved in numerous research projects, for example, in the Murphy group, the synthesis of new ruthenium-ruthenium and ruthenium-rhenium tetrametallic dendrimers with previously under-utilized bridging ligands for investigating metal-metal communication.
Research Projects
Redox active (“non-innocent”) ligands are becoming increasingly important partners to transition metal centers in assisting with delivering multiple electrons to substrates. One particular instance in which these ligands may provide such assistance would be the idea of “electron storage”, in that the ligand could hold some of the required electrons needed for the reaction, lessening the burden on the metal. If an iron(II) center reacting with an oxidizing substrate only has to reach iron(III) and not iron(IV) or iron(V) this would obviously allow the reaction to be much more energetically favorable, and thus faster. One such redox active ligand has been identified by the Flood group here at Indiana University called 2,6-(bis)-tetrazinylpyridine (btzp). The tetrazine moieties offer unique optical and electronic properties, including an unusually low pi* orbital, giving rise to the overall redox non-innocence of btzp.
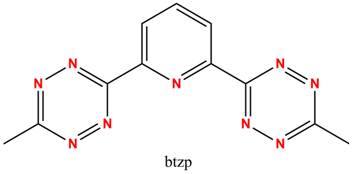
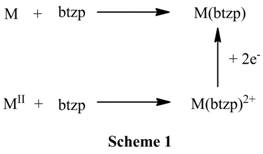
While proven to be redox active alone in solution and complexed to a metal in the form of Fe(btzp)2,I am currently working on developing, characterizing, and eventually applying 1:1 metal complexes with btzp in the form of M(btzp). M(btzp) is much more desirable because multiple empty metal orbitals are available for substrate binding, and we would predict high reactivity for these complexes. Currently, the metals under my investigation are Fe, Ru, and Zn. Fe and Zn are attractive choices because they allow exploration of the purely redox active character of the ligand with a redox inert metal (Zn) and the abundance in the earth’s crust of Fe makes it a hot target for catalyst synthesizers. One scheme for developing these complexes is shown below (scheme 1 where M = Zn, Ru). Ru is attractive because of its relative ease of handling and characterization (almost exclusively low-spin and diamagnetic), whilst being an electronic analog to Fe. Besides complete characterization, my plan for these complexes is the reduction of CO2and N2as we continue to discover more and more about the potential and hidden power stored in redox active ligands.
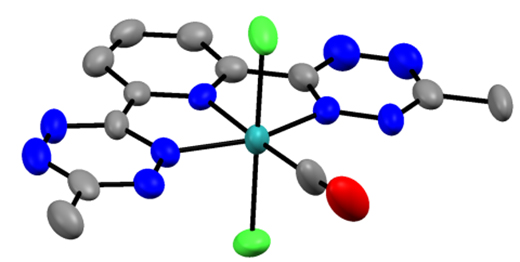
To-date I have synthesized and characterized (btzp)RuCl2(CO), incorporating the CO ligand as a reporter, which shows that btzp is the most electron withdrawing pincer ligand yet discovered, and thus with the highest CO stretching frequency observed.
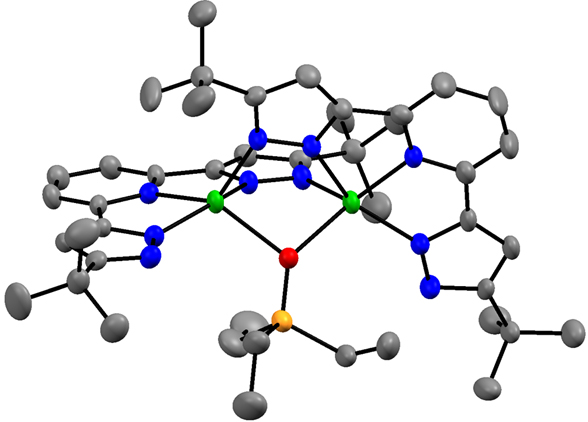
In addition, I have developed the 3d metal chemistry of the 2,6 bis-pyrazolyl pyridine chemistry of Fe and Co. This ligand is highly proton-responsive and redox active, leading to rich possibilities in small molecule activation. I have established the general tendency of the deprotonated version of these pyrazolates to aggregate, including any available oxo ligands. I have also shown that hydride reagents convert the cobalt complex to an aggregated species of Co(I), in green, to give an unprecedented oxo (in red) complex of this four coordinate
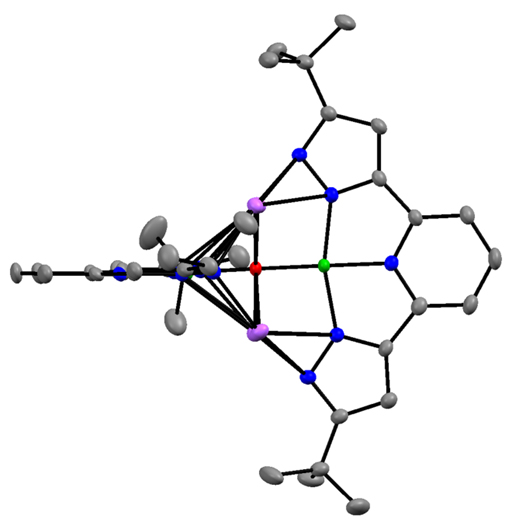
monovalent cobalt. I have a low-valent but highly fragile reduced cobalt species which I am currently characterizing as a possible reagent to reduce N2. I have also shown that LCo(PEt3)2 reacts rapidly with N2O to oxidize all phosphine to liberate OPEt3, and L2Co2(m-OPEt3), the latter containing an unprecedented bridging phosphine oxide. I already have obtained LFe(DMAP)3, where DMAP is para-dimethylamino pyridine, and this reacts rapidly with CO2 to give a carbonate product.