Biography
I was born in San Salvador, El Salvador one morning of Feb 1974. From 1992 I attended the University of El Salvador at the School of Chemistry and Pharmacy for three years. After my third year I became an undergraduate Fulbright scholar and came to the University of Louisville, Kentucky where I graduated with a BS in Chemistry with concentration in Biotechnology. After one and a half years working in El Salvador I returned to Louisville for my PhD under supervision of Prof. Dorothy Gibson. For my thesis work I focused on the synthesis of Ru polypyridyl complexes that were proposed intermediates in electrochemical reductions of CO2. During this time I developed a deep interest in the area of small molecule activation for energy conversion purposes. After finishing my PhD, I continued to work in the labs of Prof. Gibson for almost two years and in June 2007 I joined the research group of Prof. Mu-Hyun Baik as a postdoctoral researcher. I have worked on the computational analysis of proposed CO2 catalysts to intelligently design the next best catalyst with the goal of using CO2 for the synthesis of fuels. More recently I have started collaborations with Prof. Kenneth Caulton and Prof. Daniel Mindiola for quantum chemical modeling of their chemical reactions.
Research Projects
In the Caulton group I have applied DFT to determine the mechanism of reaction of highly reactive (PNP)M-L (PNP = (tBu2PCH2SiMe2)2N-; M = Ni, Rh, Os; L = H, no ligand) complexes with H2, O2, CO2 and other substrates. For example, there are no complexes of the intact molecule H2 attached to a Ni(II) center, yet experiments under way at IU have now detected one.
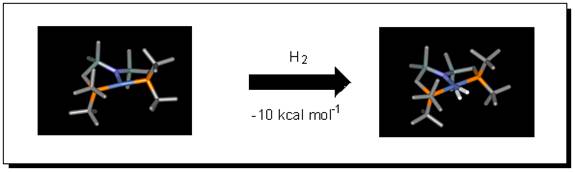
Questions I am trying to answer are how is this H2 bound to nickel, what is its energy of dissociation, and what follow-up reaction might this species do? Another unprecedented molecule made by Nick Tsvetkov in the group is (PNP)RhO; this too reacts with H2, to form simply coordinated water!
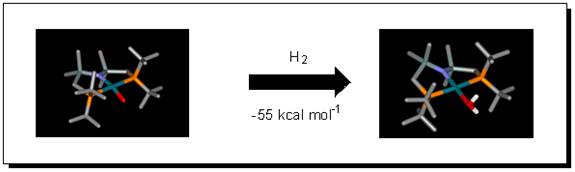
Mechanistically, how does H2 attack this molecule? Is there ever a Rh/H bond formed in any intermediate, or does H2 truly attack directly on the oxo ligand?
Quite different reactivity is the condensation of CO2, my favorite molecule, with (PNP)RhO. This forms a carbonate complex, whose geometry I have established by DFT calculations, but what is its mechanism of formation?
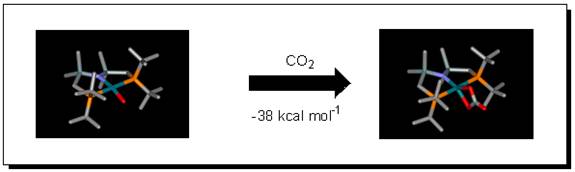
In favorable cases, the results of my calculations challenge the experimentalists to do new hypothesis-testing studies, often at low temperature.

