Biography
I am from Michigan and I graduated from Grand Valley State University with a B.S. in chemistry. As an undergraduate at GVSU, I worked for Professor Richard Lord, doing computational chemistry, primarily analyzing the electronic structure of early transition metal complexes. I spent a summer in a synthetic lab at Wayne State University, working under Professor Stanislav Groysman designing and synthesizing low-coordinate transition metal alkoxide complexes for the activation of small molecules. That summer research experience was the driving force in my decision to continue synthesis in graduate school. This brought me to Indiana University to work in Ken Caulton’s group to continue exploring catalytic ways to activate small molecules and recycle them into more useful starting materials.
Research Projects
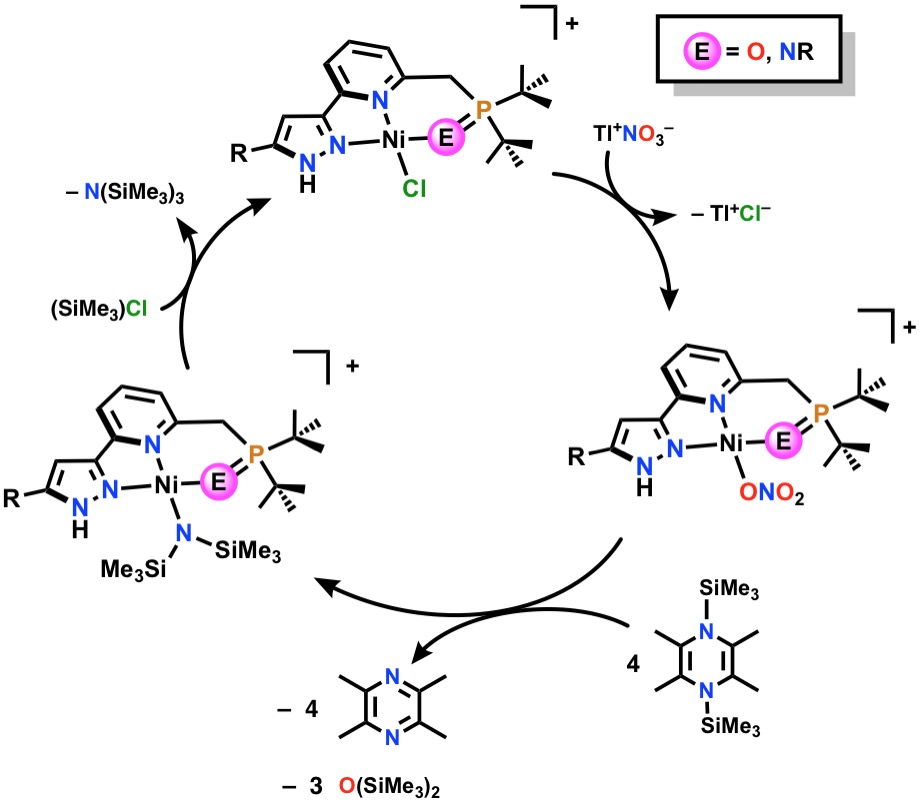
My research focuses on the design and synthesis of transition metal compounds that can bind and aide in subsequent nitrate reduction. Nitrates are harmful environmental pollutants that contain nitrogen in its highest oxidation state—N+5. I have successfully bound nitrate to both cobalt and nickel supported by a novel pincer ligand, PNNH, which is comprised of a central pyridine donor flanked by a phosphine and pyrazolyl arm. In general, our group has employed an unusual reductant, coined the “Mashima reagent” to reduce these high-oxidation state nitrogen oxyanions. My work with (PNN)Ni(NO3) and the Mashima reagent thus far has demonstrated complete deoxygenation of the nitrate ligand to give a transient nitride, which is then intramolecularly reduced by the phosphine arm in the pincer. The low coordination number of the resulting nickel leads to aggregation, which is depicted below. This demonstrates total reduction of the nitrogen from +5 to -3 in the product cluster.
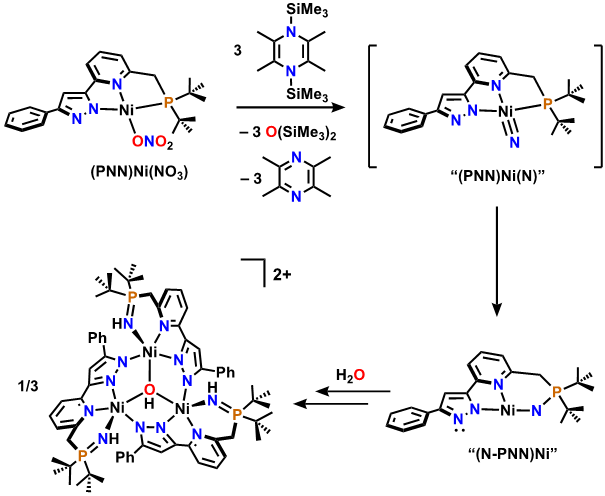
My work now focuses on catalytic nitrate reduction, which will begin with a “protected” phosphine pincer, illustrated below. Once the vulnerability of the pincer is turned off, we can imagine that following complete deoxygenation, reaction of the transient nitride with an additional equivalent of the Mashima reagent will reductively silylate the high-energy intermediate, giving rise to the seemingly conventional product (PNNH)Ni(N(SiMe3)2. This proposed catalytic cycle is depicted below.