Biography
I was born and raised in Cincinnati, Ohio. I received my B.S. in chemistry from the University of Cincinnati in 2017, where I worked in the lab of Dr. Bill Connick. When I’m not in the lab, I like to hike with my dog, run, go to the ballet, read, and even snorkel if I can. I chose Indiana University because of the welcoming graduate students in the department and my strong interest in several different advisors’ research, as well as the beauty of the campus and hiking trails around town. I’m interested in organic and inorganic synthesis for the catalysis of small molecule conversions.
Research Projects
Nitrates and nitrites exist as a waste product in water that result mainly from fertilizer runoff and septic tank leakage. If not removed from runoff, nitrates cause excess algae growth in rivers and oceans, draining oxygen from the water and killing fish and other vital organisms. Eutrophication is estimated to cause $2.2 billion of damage each year.1
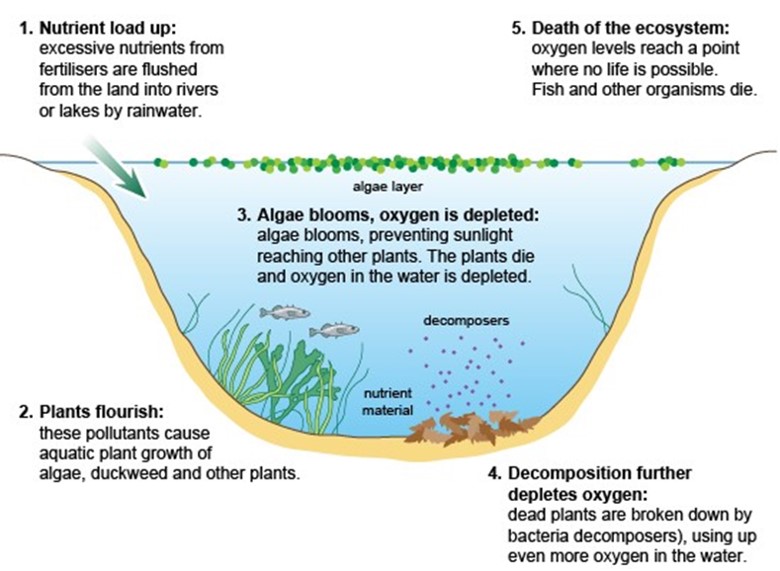
Existing nitrate treatment options such as ion exchange, reverse osmosis, and electrodialysis only include nitrate removal, which generates a harmful waste brine.2 Chemical nitrate reduction does not produce this hazardous waste stream and produces value-added products.

In my research, I am trying to develop a chromium-based electrocatalyst that can reduce nitrate to downstream reduction products in aqueous environments. Chromium is a relatively cheap, earth-abundant metal, which makes it attractive for this purpose. It has several oxidation states, which gives it the needed electron transfer chemistry. Cyclam is a tetradentate ligand which may provide structure for the chromium complex’s interaction with nitrate.3
I have shown that cis– and trans-[Cr(cyclam)Cl2]Cl are able to selectively (and quantitatively, in the case of the trans isomer) reduce nitrate to nitrite in aqueous conditions at a mercury pool electrode.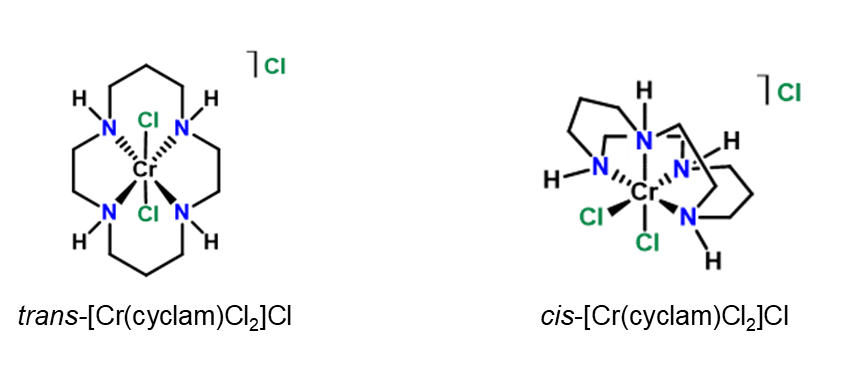
The isomers exhibit different electrochemical behavior at a mercury pool electrode, while sharing similar behavior at a glassy carbon electrode. The trans complex is reduced at a less negative potential due to a suspected adsorptive interaction between this isomer and the mercury surface, leading to a less negative onset potential for nitrate anion reduction than with the cis complex. The mechanism of this interaction is being explored experimentally and computationally, which should provide more insight.
- M. F. Chislock, E. Doster, R. A. Zitomer and A. Wilson, E., Nature Education, 2013, 4.
- V. B. Jensen, J. L. Darby, C. Seidel and C. Gorman, Drinking Water Treatment for Nitrate, University of California, Davis, 2012.
- N. A. Kane-Maguire, K. C. Wallace and D. B. Miller, Inorganic Chemistry, 1985, 24, 597-605.